Sunday Poster Session
Category: Biliary/Pancreas
P0158 - Weight Loss, Unexpected Cost: GLP-1 Receptor Agonist-Associated Acalculous Cholecystitis
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Kaitlyn Ghassemi, DO (she/her/hers)
UHS SoCal MEC
Temecula, CA
Presenting Author(s)
Kaitlyn Ghassemi, DO1, Ryan Burd, DO2, Ryan Gilbertson, MD2
1UHS SoCal MEC, Temecula, CA; 2Southwest Healthcare MEC, Temecula, CA
Introduction: GLP-1 receptor agonists (GLP-1 RAs) are widely used for diabetes and weight loss due to their effects on insulin secretion, gastric emptying, and appetite suppression. While gastrointestinal side effects are common, biliary complications such as cholelithiasis and cholecystitis are less frequently reported. Acalculous cholecystitis, an acute necroinflammatory disease of the gallbladder occurring in the absence of gallstones, is a particularly rare consequence of GLP-1 RA use. We present a case of acalculous cholecystitis in a patient on chronic tirzepatide (Zepbound), adding to growing evidence of GLP-1 RA–associated biliary disease in the absence of traditional risk factors.
Case Description/
Methods: A 56-year-old male with a past medical history of hypertension, hyperlipidemia and gout presented to the emergency department with 3 days of right upper quadrant abdominal pain, nausea, vomiting and fever. For the past year, the patient was taking Zepbound 7.5mg weekly for weight loss, stating that he experienced progressive abdominal discomfort since initiation. Abnormal vitals upon admission were body temperature 100.6F and heart rate of 109. Abnormal lab values included white blood cells of 15.52, sodium 125. CT abdomen showed evidence of acalculous cholecystitis. Confirmatory HIDA scan returned positive for cholecystitis without choledocholithiasis. The patient was diagnosed with acalculous cholecystitis and underwent cholecystectomy without complications. Blood cultures indicated E. coli bacteremia for which the patient received IV Rocephin with oral antibiotics prescribed upon discharge. The patient was advised cessation of Zepbound weekly injection until after follow-up with their primary care provider.
Discussion: Due to decreased bowel motility caused by GLP-1 RA effects on myenteric neurons and vagus parasympathetic activity, commonly documented side effects of use include ileus, nausea, vomiting and altered oral metabolism. Less commonly documented effects from Phase 3 trials include pancreatitis, cholelithiasis and cholecystitis. Acalculous cholecystitis is an exceedingly rare consequence, described by only two other published case reports to date. This case contributes another example of this rare association and highlights the extended biliary risks with GLP-1 RA use. While the mechanism of interaction is currently unknown, GLP-1 RAs may inhibit peristalsis of the biliary ducts in a similar manner to the bowel, making users more prone to biliary stasis and acalculous cholecystitis.
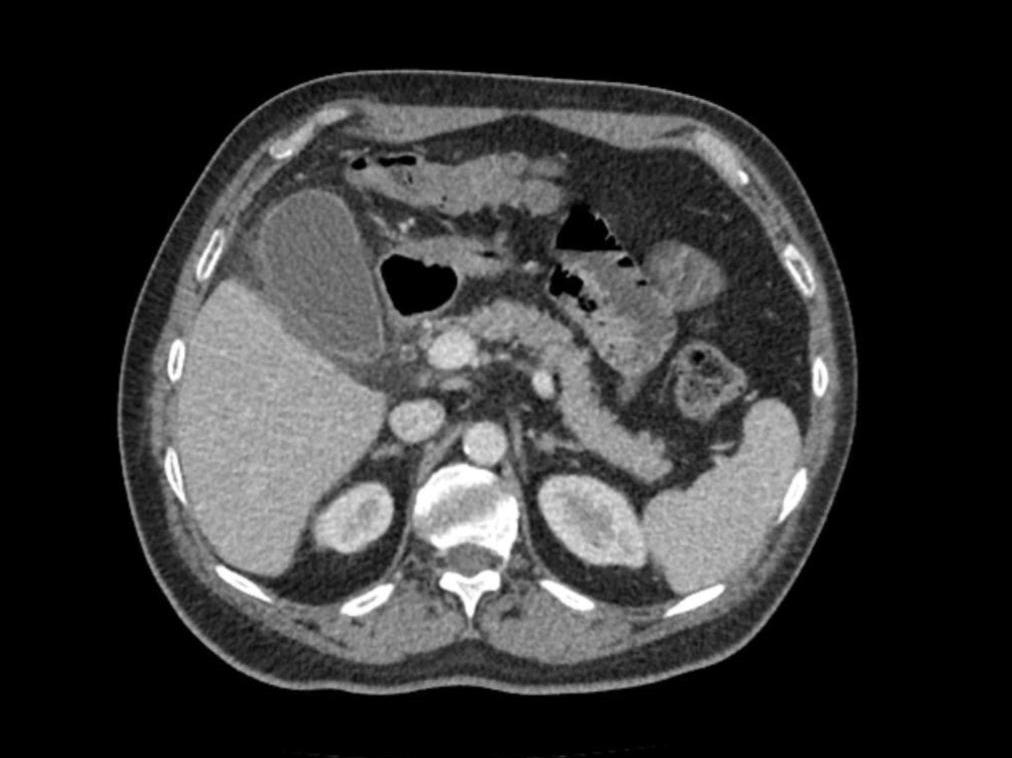
Figure: Figure 1. Computed tomography of abdomen/pelvis demonstrating mild hepatic steatosis, splenomegaly and a distended gallbladder without visualized gallstones and with pericholecystic fat stranding commonly seen in acalculous cholecystitis.
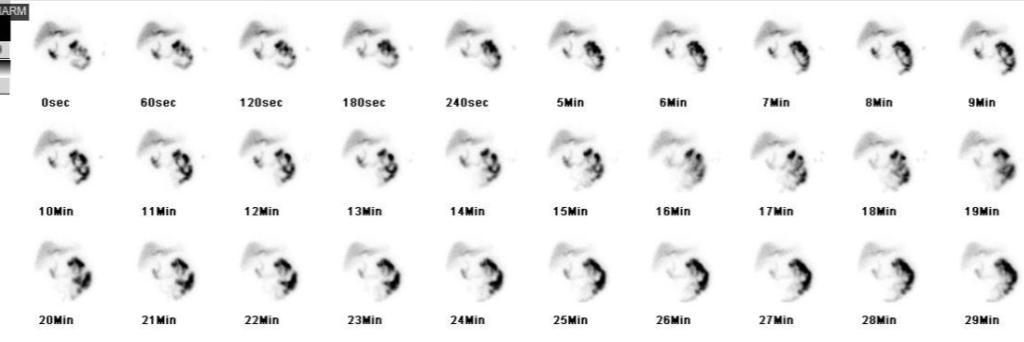
Figure: Figure 2. Hepatobiliary iminodiacetic acid scan demonstrating absence of radiotracer uptake in the gallbladder without evidence of common bile duct obstruction, indicative of cholecystitis.
Disclosures:
Kaitlyn Ghassemi indicated no relevant financial relationships.
Ryan Burd indicated no relevant financial relationships.
Ryan Gilbertson indicated no relevant financial relationships.
Kaitlyn Ghassemi, DO1, Ryan Burd, DO2, Ryan Gilbertson, MD2. P0158 - Weight Loss, Unexpected Cost: GLP-1 Receptor Agonist-Associated Acalculous Cholecystitis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1UHS SoCal MEC, Temecula, CA; 2Southwest Healthcare MEC, Temecula, CA
Introduction: GLP-1 receptor agonists (GLP-1 RAs) are widely used for diabetes and weight loss due to their effects on insulin secretion, gastric emptying, and appetite suppression. While gastrointestinal side effects are common, biliary complications such as cholelithiasis and cholecystitis are less frequently reported. Acalculous cholecystitis, an acute necroinflammatory disease of the gallbladder occurring in the absence of gallstones, is a particularly rare consequence of GLP-1 RA use. We present a case of acalculous cholecystitis in a patient on chronic tirzepatide (Zepbound), adding to growing evidence of GLP-1 RA–associated biliary disease in the absence of traditional risk factors.
Case Description/
Methods: A 56-year-old male with a past medical history of hypertension, hyperlipidemia and gout presented to the emergency department with 3 days of right upper quadrant abdominal pain, nausea, vomiting and fever. For the past year, the patient was taking Zepbound 7.5mg weekly for weight loss, stating that he experienced progressive abdominal discomfort since initiation. Abnormal vitals upon admission were body temperature 100.6F and heart rate of 109. Abnormal lab values included white blood cells of 15.52, sodium 125. CT abdomen showed evidence of acalculous cholecystitis. Confirmatory HIDA scan returned positive for cholecystitis without choledocholithiasis. The patient was diagnosed with acalculous cholecystitis and underwent cholecystectomy without complications. Blood cultures indicated E. coli bacteremia for which the patient received IV Rocephin with oral antibiotics prescribed upon discharge. The patient was advised cessation of Zepbound weekly injection until after follow-up with their primary care provider.
Discussion: Due to decreased bowel motility caused by GLP-1 RA effects on myenteric neurons and vagus parasympathetic activity, commonly documented side effects of use include ileus, nausea, vomiting and altered oral metabolism. Less commonly documented effects from Phase 3 trials include pancreatitis, cholelithiasis and cholecystitis. Acalculous cholecystitis is an exceedingly rare consequence, described by only two other published case reports to date. This case contributes another example of this rare association and highlights the extended biliary risks with GLP-1 RA use. While the mechanism of interaction is currently unknown, GLP-1 RAs may inhibit peristalsis of the biliary ducts in a similar manner to the bowel, making users more prone to biliary stasis and acalculous cholecystitis.

Figure: Figure 1. Computed tomography of abdomen/pelvis demonstrating mild hepatic steatosis, splenomegaly and a distended gallbladder without visualized gallstones and with pericholecystic fat stranding commonly seen in acalculous cholecystitis.

Figure: Figure 2. Hepatobiliary iminodiacetic acid scan demonstrating absence of radiotracer uptake in the gallbladder without evidence of common bile duct obstruction, indicative of cholecystitis.
Disclosures:
Kaitlyn Ghassemi indicated no relevant financial relationships.
Ryan Burd indicated no relevant financial relationships.
Ryan Gilbertson indicated no relevant financial relationships.
Kaitlyn Ghassemi, DO1, Ryan Burd, DO2, Ryan Gilbertson, MD2. P0158 - Weight Loss, Unexpected Cost: GLP-1 Receptor Agonist-Associated Acalculous Cholecystitis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
