Sunday Poster Session
Category: Biliary/Pancreas
P0003 - A Retrospective Single Center Case Series of Prophylactic Rectal Indomethacin Use During Endoscopic Ultrasound Guided Pancreatic Biopsy: A Pilot Study
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
- ML
Muhddesa Lakhana, DO
Mount Sinai South Nassau,Icahn School of Medicine at Mount Sinai
Oceanside, NY
Presenting Author(s)
Muhddesa Lakhana, DO1, Pranay Srivastava, MD2, Neal Shah, DO1, Haidar Khan, MD1, Maria Chimarios, DO3, Sharon Slomovich, MD1, Jonathan Reyes, MD1, Rashmi Advani, MD1, Sadat Iqbal, MD1, Bhanu Singh, MD1, Frank G. Gress, MD, MBA1
1Mount Sinai South Nassau,Icahn School of Medicine at Mount Sinai, Oceanside, NY; 2NYU Langone Health, East Patchogue, NY; 3NYIT College of Osteopathic Medicine, Oceanside, NY
Introduction: Endoscopic retrograde cholangiopancreatography (ERCP) is a known cause of post-procedure pancreatitis, for which prophylactic indomethacin is commonly used. Similarly, pancreatitis can occur after Endoscopic Ultrasound (EUS) with fine-needle aspiration (FNA) or fine-needle biopsy (FNB) of the pancreas. However, there is limited data evaluating the use of indomethacin to prevent pancreatitis after a biopsy.
This is a retrospective study looking at the role of indomethacin in pancreatic biopsy.
Methods: We conducted a single-center retrospective study evaluating patients who underwent EUS-guided FNA or FNB of the pancreas. Indomethacin administration was at the discretion of the endoscopist. Data collected included procedural indications, lesion characteristics, needle type, number of passes, communication with the pancreatic duct, and post-procedural complications. Patients were monitored for symptoms and instructed to report any post-procedural pain. A total of 80 patients were included: 40 received rectal indomethacin and the other 40 patients did not.
Results: A total of 80 patients were included. The various indications included mass, cyst, stricture, and parenchymal abnormalities. Both cysts and masses were either aspirated and/or biopsied. Figure 1 includes the patient who did not have pancreatitis after the post-procedure. Figure 2 breaks down the characteristics of the patients who had pancreatitis post- procedurally. Four cases of post procedural pancreatitis were noted: 3 in patients who did not receive indomethacin and one patient who received indomethacin.The odds ratio was 4.33 (95% CI: 0.46–40.61; p = 0.359).
Discussion: This pilot study suggests that indomethacin may reduce the incidence of post-EUS pancreatitis, though the difference was not statistically significant. Communication with the pancreatic duct may be a contributing risk factor. No major complications were observed in either group.
Four patients developed pancreatitis. The three cases that did not receive indomethacin had a mass or a cyst that was in communication with the pancreatic duct and were hospitalized for pancreatitis. In the patient who received indomethacin, it is unclear whether there was communication with the pancreatic duct and symptoms improved in 24 hours.
Limitations include small sample size and variability in documentation. A larger, prospective randomized trial is currently underway to further investigate the role of indomethacin in patients undergoing pancreatic FNA/FNB.
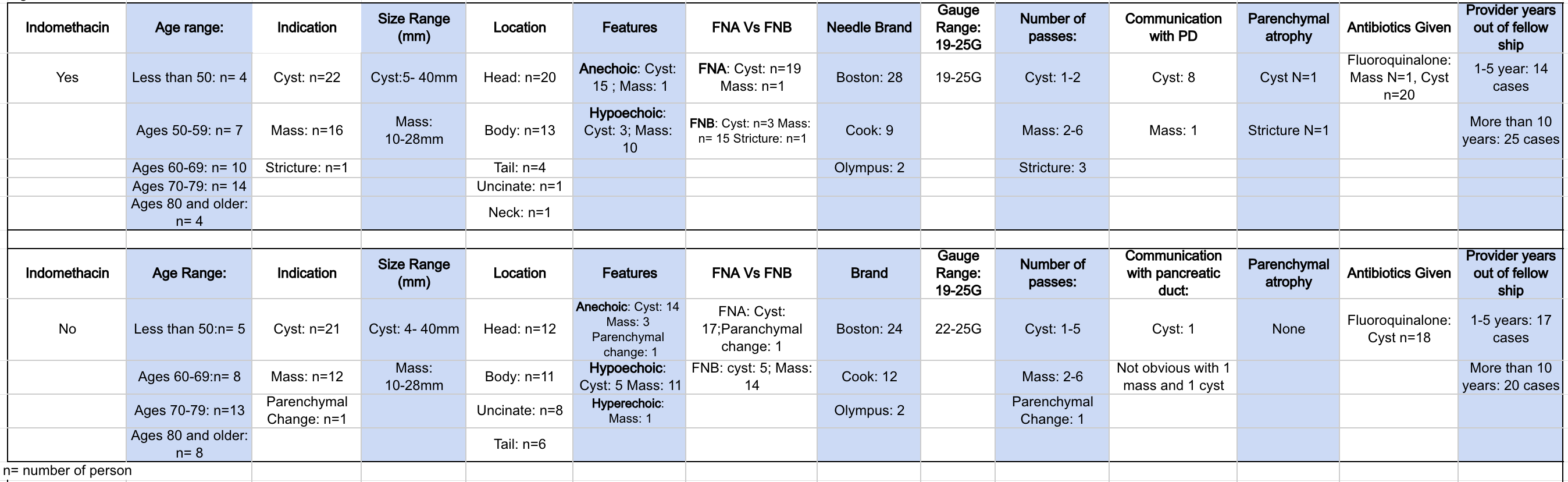
Figure: Figure 1: Characteristics of patients: No pancreatitis post procedurally.
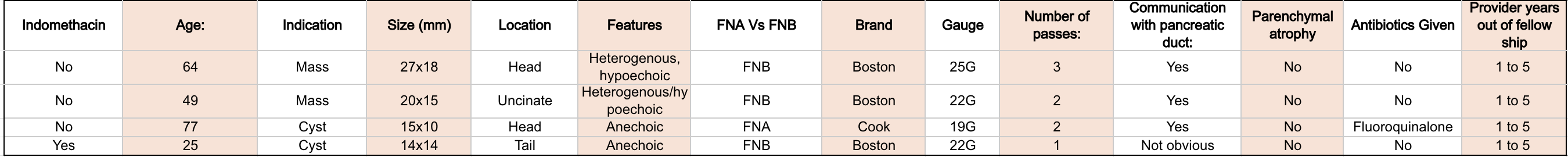
Figure: Figure 2: Characteristics of patients: Pancreatitis post procedurally.
Disclosures:
Muhddesa Lakhana indicated no relevant financial relationships.
Pranay Srivastava indicated no relevant financial relationships.
Neal Shah indicated no relevant financial relationships.
Haidar Khan indicated no relevant financial relationships.
Maria Chimarios indicated no relevant financial relationships.
Sharon Slomovich indicated no relevant financial relationships.
Jonathan Reyes indicated no relevant financial relationships.
Rashmi Advani indicated no relevant financial relationships.
Sadat Iqbal indicated no relevant financial relationships.
Bhanu Singh indicated no relevant financial relationships.
Frank Gress indicated no relevant financial relationships.
Muhddesa Lakhana, DO1, Pranay Srivastava, MD2, Neal Shah, DO1, Haidar Khan, MD1, Maria Chimarios, DO3, Sharon Slomovich, MD1, Jonathan Reyes, MD1, Rashmi Advani, MD1, Sadat Iqbal, MD1, Bhanu Singh, MD1, Frank G. Gress, MD, MBA1. P0003 - A Retrospective Single Center Case Series of Prophylactic Rectal Indomethacin Use During Endoscopic Ultrasound Guided Pancreatic Biopsy: A Pilot Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Mount Sinai South Nassau,Icahn School of Medicine at Mount Sinai, Oceanside, NY; 2NYU Langone Health, East Patchogue, NY; 3NYIT College of Osteopathic Medicine, Oceanside, NY
Introduction: Endoscopic retrograde cholangiopancreatography (ERCP) is a known cause of post-procedure pancreatitis, for which prophylactic indomethacin is commonly used. Similarly, pancreatitis can occur after Endoscopic Ultrasound (EUS) with fine-needle aspiration (FNA) or fine-needle biopsy (FNB) of the pancreas. However, there is limited data evaluating the use of indomethacin to prevent pancreatitis after a biopsy.
This is a retrospective study looking at the role of indomethacin in pancreatic biopsy.
Methods: We conducted a single-center retrospective study evaluating patients who underwent EUS-guided FNA or FNB of the pancreas. Indomethacin administration was at the discretion of the endoscopist. Data collected included procedural indications, lesion characteristics, needle type, number of passes, communication with the pancreatic duct, and post-procedural complications. Patients were monitored for symptoms and instructed to report any post-procedural pain. A total of 80 patients were included: 40 received rectal indomethacin and the other 40 patients did not.
Results: A total of 80 patients were included. The various indications included mass, cyst, stricture, and parenchymal abnormalities. Both cysts and masses were either aspirated and/or biopsied. Figure 1 includes the patient who did not have pancreatitis after the post-procedure. Figure 2 breaks down the characteristics of the patients who had pancreatitis post- procedurally. Four cases of post procedural pancreatitis were noted: 3 in patients who did not receive indomethacin and one patient who received indomethacin.The odds ratio was 4.33 (95% CI: 0.46–40.61; p = 0.359).
Discussion: This pilot study suggests that indomethacin may reduce the incidence of post-EUS pancreatitis, though the difference was not statistically significant. Communication with the pancreatic duct may be a contributing risk factor. No major complications were observed in either group.
Four patients developed pancreatitis. The three cases that did not receive indomethacin had a mass or a cyst that was in communication with the pancreatic duct and were hospitalized for pancreatitis. In the patient who received indomethacin, it is unclear whether there was communication with the pancreatic duct and symptoms improved in 24 hours.
Limitations include small sample size and variability in documentation. A larger, prospective randomized trial is currently underway to further investigate the role of indomethacin in patients undergoing pancreatic FNA/FNB.

Figure: Figure 1: Characteristics of patients: No pancreatitis post procedurally.

Figure: Figure 2: Characteristics of patients: Pancreatitis post procedurally.
Disclosures:
Muhddesa Lakhana indicated no relevant financial relationships.
Pranay Srivastava indicated no relevant financial relationships.
Neal Shah indicated no relevant financial relationships.
Haidar Khan indicated no relevant financial relationships.
Maria Chimarios indicated no relevant financial relationships.
Sharon Slomovich indicated no relevant financial relationships.
Jonathan Reyes indicated no relevant financial relationships.
Rashmi Advani indicated no relevant financial relationships.
Sadat Iqbal indicated no relevant financial relationships.
Bhanu Singh indicated no relevant financial relationships.
Frank Gress indicated no relevant financial relationships.
Muhddesa Lakhana, DO1, Pranay Srivastava, MD2, Neal Shah, DO1, Haidar Khan, MD1, Maria Chimarios, DO3, Sharon Slomovich, MD1, Jonathan Reyes, MD1, Rashmi Advani, MD1, Sadat Iqbal, MD1, Bhanu Singh, MD1, Frank G. Gress, MD, MBA1. P0003 - A Retrospective Single Center Case Series of Prophylactic Rectal Indomethacin Use During Endoscopic Ultrasound Guided Pancreatic Biopsy: A Pilot Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
