Sunday Poster Session
Category: Colon
P0297 - Pan-Gastrointestinal Immune-Related Adverse Events: Characterization and Outcomes

Maria Julia M. N Santos, MD (she/her/hers)
MD Anderson Cancer Center
Houston, TX
Presenting Author(s)
1MD Anderson Cancer Center, Houston, TX; 2University of Texas MD Anderson Cancer Center, Houston, TX
Introduction:
Gastrointestinal (GI) immune-related adverse events (irAEs) are among the most common side effects experienced by cancer patients treated with immune checkpoint inhibitors (ICI). While upper GI toxicities, such as esophagitis, gastritis, and enteritis, as well as lower tract GI toxicities like diarrhea and colitis, have gained increased recognition, the diffuse gastrointestinal presentation and overlapping symptoms of these conditions are not well described in the current literature.This study aims to characterize these presentations and to outline the management and outcomes for affected patients.
Methods:
This is a retrospective analysis of 1176 patients with diagnosis of upper tract and/or lower tract GI-irAEs, categorized in 3 groups: isolated upper GI-irAE(n=228), isolated lower GI-irAEs(n=673),or concurrent upper and lower GI-irAEs(n=140). Additionally, a subset of 23 patients with concomitant esophagitis, gastritis, enteritis and colitis were classified as pan-gastrointestinal toxicity. Clinical characteristics, endoscopic findings, management and outcomes were compared across groups.
Results:
Combination ICI use was more frequent among patients with diffuse GI toxicity (36.4%) compared to those with isolated upper (5.5%) or lower (37.6%) GI involvement (p< 0.001). The number of ICI doses before toxicity was similar across the three groups. Patients with concurrent toxicity had higher rates of grade 3–4 events (73.0%), hospitalization (71.4%), steroid use (74.3%), SIT (52.1%), and FMT (5.7%) (all p< 0.001). Clinical resolution occurred in 79.3%, with recurrence in 15.7%.12.9% resumed ICI.
Among patients with pan-GI toxicity,52.2% had received combination ICI.Mucosal ulcerations were observed on both upper endoscopy (21.7%) and colonoscopy (21.7%).Hospitalization was required in 78.3%, steroids in 87%,SIT in 69.6%,and FMT in 26.1%.Resolution was achieved in 91.3%, though recurrence occurred in 13%.ICU admission and all-cause mortality were 4.3% and 30.4%, respectively.13% were able to resume ICI therapy after the event.
Discussion: This is the largest cohort characterizing patients with concurrent upper and lower GI-irAEs, and the first to describe patients with pan-GI toxicity. Concurrent upper and lower GI irAEs, including pan-GI presentations, represent a severe form of ICI toxicity marked by high-grade inflammation, extensive ulceration, and frequent need for immunosuppressive therapy. These patients require prompt recognition and may benefit from early escalation of treatment.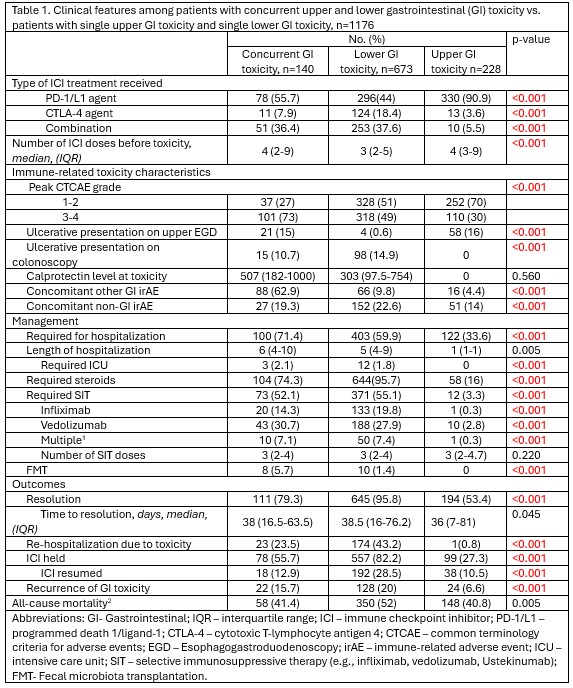
Figure: Table 1. Clinical features among patients with concurrent upper and lower gastrointestinal (GI) toxicity vs. patients with single upper GI toxicity and single lower GI toxicity, n=1176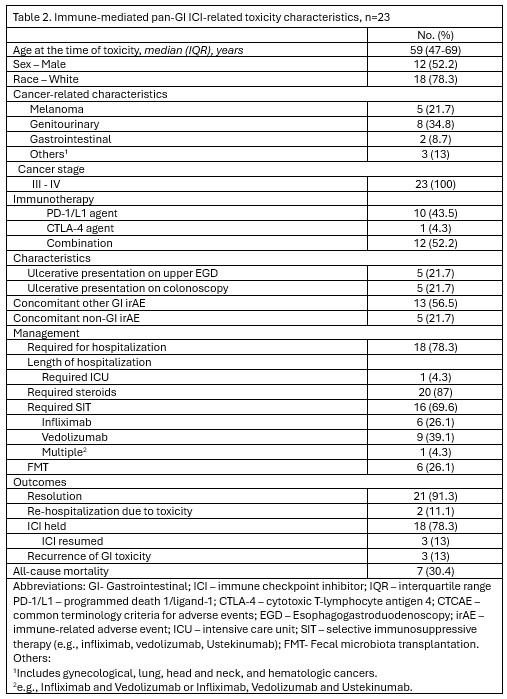
Figure: Table 2. Immune-mediated pan-GI ICI-related toxicity characteristics, n=23
Disclosures:
Maria Julia Santos indicated no relevant financial relationships.
Carolina Cruz indicated no relevant financial relationships.
Sharada Wali indicated no relevant financial relationships.
Krishnavathana Varatharajalu indicated no relevant financial relationships.
Yinghong Wang indicated no relevant financial relationships.
Maria Julia M. N.. Santos, MD1, Carolina Cruz, MD2, Sharada Wali, MBBS, MPH2, Krishnavathana Varatharajalu, MD1, Yinghong Wang, MD, PhD, MS2. P0297 - Pan-Gastrointestinal Immune-Related Adverse Events: Characterization and Outcomes, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
