Sunday Poster Session
Category: Colorectal Cancer Prevention
P0527 - Development and Validation of Colon Polyp Surveillance Patient-Reported Outcomes and Experience Measures
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Audrey Calderwood, MD, MS (she/her/hers)
Dartmouth Hitchcock Medical Center
Lebanon, NH
Presenting Author(s)
Sidney May, PhD1, Audrey Calderwood, MD, MS2, Erin Knight, PhD1, Nancy Boyer, PhD, MSW1, Karen Schifferdecker, PhD, MPH1
1Geisel School of Medicine, Lebanon, NH; 2Dartmouth Hitchcock Medical Center, Lebanon, NH
Introduction: Polyp surveillance is the most common indication for colonoscopy in adults ≥65 years accounting for almost 6 million colonoscopies in 2024. Patient-reported outcome (PROs) and experience measures (PREMs) are essential in assessing outcomes important to patients in comparative effectiveness research and beyond. While there are validated measures for colorectal cancer screening, there are no existing instruments for patients undergoing polyp surveillance. Our aim was to develop and validate PROs/PREMs for this purpose.
Methods: We used an instrument development mixed methods design to identify PRO/PREM domains related to polyp surveillance and evaluate the psychometric properties of PRO/PREM items. Phase 1 involved semi-structured interviews with 14 older adults with colon polyps and 9 primary care physicians. Results guided development of items, refined through cognitive interviews (N=10). In Phase 2, we distributed the final 16-item PRO/PREM questionnaire (Table 1) to 450 older adults with colon polyps. We used exploratory factor analyses (EFA) with oblique oblimin rotation to examine the factor structure, and confirmatory factor analyses (CFA; N=466) to confirm. Test-retest reliability was assessed with a subset of participants (N=155).
Results: Interviews revealed 6 PRO/PREM domains: Test Satisfaction, Test Convenience, Testing Complications, Assistance Needed, Confidence in Test, and Cancer Worry. EFA results supported a 3-factor solution with 11 items (Test Satisfaction, Cancer Worry Reduction, and General Cancer Worry). The CFA results did not support this 3-factor structure (CFI=0.96; RMSEA=0.085; SRMR=0.081) and we conducted 3 re-specifications based on local model misfit and theoretical considerations. We chose a 1-factor, 4-item model of Test Satisfaction (CFI=0.99; RMSEA=0.095; SRMR=0.038; Figure 1). Test-retest reliability for this composite Test Satisfaction score was good (ICC=0.79). Test-retest reliability was moderate for 12 of the individual PRO/PREM items and poor for 3 items.
Discussion: For patients undergoing polyp surveillance, test satisfaction is captured well through questions around overall satisfaction, convenience, difficulty, and willingness to complete again. Trust in testing, potential harms, and cancer worry are also meaningful and operate most reliably as standalone items. Collection of PRO/PREMs around polyp surveillance is feasible, reliable and valid and can be used for research purposes.
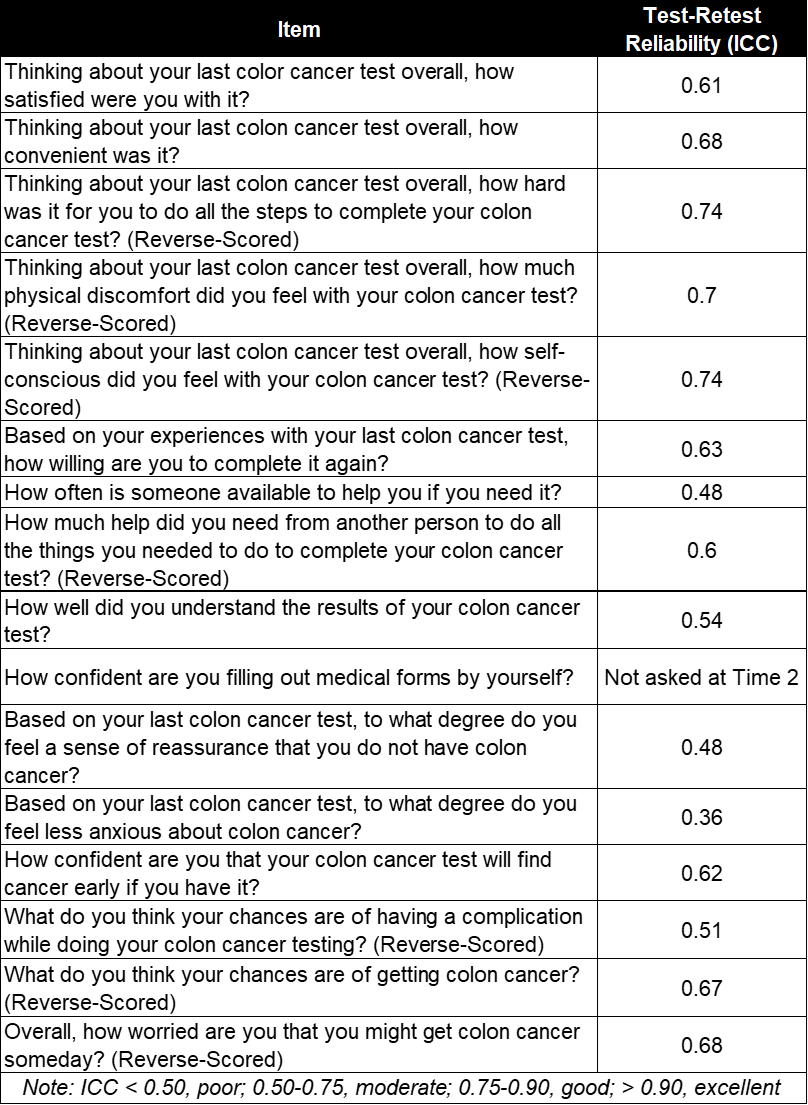
Figure: Table 1. Final 16-item PRO/PREM questionnaire regarding colon polyp surveillance distributed to 450 older adults with colon polyps
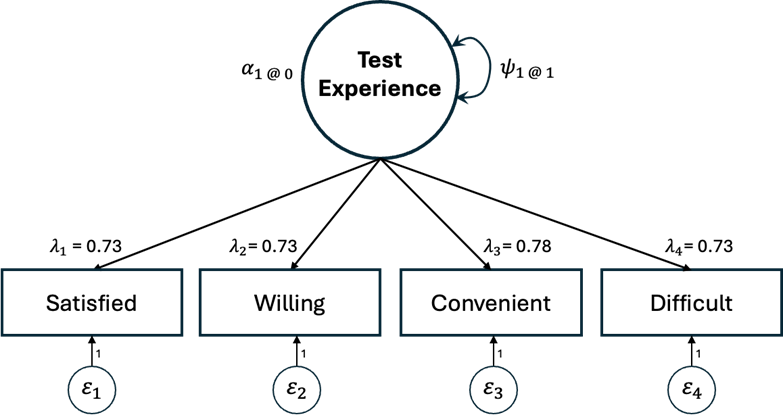
Figure: Figure 2. 1-factor, 4-item model of test satisfaction regarding colon polyp surveillance
Disclosures:
Sidney May indicated no relevant financial relationships.
Audrey Calderwood indicated no relevant financial relationships.
Erin Knight indicated no relevant financial relationships.
Nancy Boyer indicated no relevant financial relationships.
Karen Schifferdecker indicated no relevant financial relationships.
Sidney May, PhD1, Audrey Calderwood, MD, MS2, Erin Knight, PhD1, Nancy Boyer, PhD, MSW1, Karen Schifferdecker, PhD, MPH1. P0527 - Development and Validation of Colon Polyp Surveillance Patient-Reported Outcomes and Experience Measures, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Geisel School of Medicine, Lebanon, NH; 2Dartmouth Hitchcock Medical Center, Lebanon, NH
Introduction: Polyp surveillance is the most common indication for colonoscopy in adults ≥65 years accounting for almost 6 million colonoscopies in 2024. Patient-reported outcome (PROs) and experience measures (PREMs) are essential in assessing outcomes important to patients in comparative effectiveness research and beyond. While there are validated measures for colorectal cancer screening, there are no existing instruments for patients undergoing polyp surveillance. Our aim was to develop and validate PROs/PREMs for this purpose.
Methods: We used an instrument development mixed methods design to identify PRO/PREM domains related to polyp surveillance and evaluate the psychometric properties of PRO/PREM items. Phase 1 involved semi-structured interviews with 14 older adults with colon polyps and 9 primary care physicians. Results guided development of items, refined through cognitive interviews (N=10). In Phase 2, we distributed the final 16-item PRO/PREM questionnaire (Table 1) to 450 older adults with colon polyps. We used exploratory factor analyses (EFA) with oblique oblimin rotation to examine the factor structure, and confirmatory factor analyses (CFA; N=466) to confirm. Test-retest reliability was assessed with a subset of participants (N=155).
Results: Interviews revealed 6 PRO/PREM domains: Test Satisfaction, Test Convenience, Testing Complications, Assistance Needed, Confidence in Test, and Cancer Worry. EFA results supported a 3-factor solution with 11 items (Test Satisfaction, Cancer Worry Reduction, and General Cancer Worry). The CFA results did not support this 3-factor structure (CFI=0.96; RMSEA=0.085; SRMR=0.081) and we conducted 3 re-specifications based on local model misfit and theoretical considerations. We chose a 1-factor, 4-item model of Test Satisfaction (CFI=0.99; RMSEA=0.095; SRMR=0.038; Figure 1). Test-retest reliability for this composite Test Satisfaction score was good (ICC=0.79). Test-retest reliability was moderate for 12 of the individual PRO/PREM items and poor for 3 items.
Discussion: For patients undergoing polyp surveillance, test satisfaction is captured well through questions around overall satisfaction, convenience, difficulty, and willingness to complete again. Trust in testing, potential harms, and cancer worry are also meaningful and operate most reliably as standalone items. Collection of PRO/PREMs around polyp surveillance is feasible, reliable and valid and can be used for research purposes.

Figure: Table 1. Final 16-item PRO/PREM questionnaire regarding colon polyp surveillance distributed to 450 older adults with colon polyps

Figure: Figure 2. 1-factor, 4-item model of test satisfaction regarding colon polyp surveillance
Disclosures:
Sidney May indicated no relevant financial relationships.
Audrey Calderwood indicated no relevant financial relationships.
Erin Knight indicated no relevant financial relationships.
Nancy Boyer indicated no relevant financial relationships.
Karen Schifferdecker indicated no relevant financial relationships.
Sidney May, PhD1, Audrey Calderwood, MD, MS2, Erin Knight, PhD1, Nancy Boyer, PhD, MSW1, Karen Schifferdecker, PhD, MPH1. P0527 - Development and Validation of Colon Polyp Surveillance Patient-Reported Outcomes and Experience Measures, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
