Sunday Poster Session
Category: GI Bleeding
P0912 - Natural History and Outcomes of Patients With Upper GI Bleeding from Tumors
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Sean Dewberry, MD
Keck School of Medicine of the University of Southern California
Los Angeles, CA
Presenting Author(s)
Sean Dewberry, MD1, Gevork Salmastyan, MD1, Sarah Sheibani, MD1, Kathy Lui, MD1, Loren Laine, MD2, John J.. Kim, MD, MS, FACG3
1Keck School of Medicine of the University of Southern California, Los Angeles, CA; 2Yale Digestive Diseases, New Haven, CT; 3Los Angeles General Medical Center, Los Angeles, CA
Introduction: Recent information evaluating the clinical course and outcomes in patients with upper GI bleeding from tumors outside clinical trials of endoscopic therapy is limited. We evaluated the outcomes of hospitalized patients with tumor-related upper GI bleeding receiving endoscopy at an urban US hospital and compared them to outcomes at the same center 10 years ago.
Methods: Consecutive patients hospitalized (6/2015-11/2024) with hematemesis, melena, and/or hematochezia receiving endoscopy were searched using ICD 9/10 codes for upper GI tumors. Procedure reports were reviewed to confirm biopsy-proven tumors without other causes of GI bleeding. Study endpoints included hemostasis at the conclusion of endoscopy and rebleeding. Rebleeding was defined as recurrent bleeding in patients without active bleeding on index endoscopy or whose bleeding stopped spontaneously or with endoscopic, interventional radiologic, or surgical intervention.
Results: 107 (mean age 58+13 years, 81 (76%) males) patients met study criteria with tumor originating from the esophagus in 13 (12%), stomach in 84 (79%), duodenum in 9 (8%), and jejunum in 1 (1%). At presentation, 62 (58%) patients did not have a prior diagnosis of malignancy and 66 (62%) had evidence of metastatic disease. Hemodynamic instability was observed in 50 (47%) patients, and 28 (26%) were on anti-thrombotic therapy. High-risk stigmata of bleeding were reported in 48 (45%) patients: active bleeding in 31 (29%) including spurting 2 (2%) and oozing 29 (27%), non-bleeding visible vessel in 3 (3%), and adherent clot in 14 (13%). Endoscopic therapy was performed in 19 (18%), including TC-325 hemostatic powder spray in 6 (6%), epinephrine injection alone in 6 (6%), hemoclips in 3 (3%), bipolar coagulation in 2 (2%), polysaccharide hemostatic powder in 1 (1%), and alcohol injection in 1 (1%). Overall, 9 (8%) patients did not attain hemostasis; 13 (13%) of 103 and 19 (19%) of 100 developed rebleeding within 3 and 7 days, respectively (Table 1). During a mean follow-up of 10.6+16.7 months, 50 (47%) patients developed rebleeding, similar to 48 (47%) of 103 from the 2005-2012 cohort (Table 2).
Discussion: Active bleeding was encountered in nearly one-third of patients with tumor-related upper GI bleeding. Most patients attained endoscopic hemostasis with or without therapy. Similar to a decade ago, initial control of bleeding was successful in most patients, but nearly a half developed long-term recurrent bleeding.
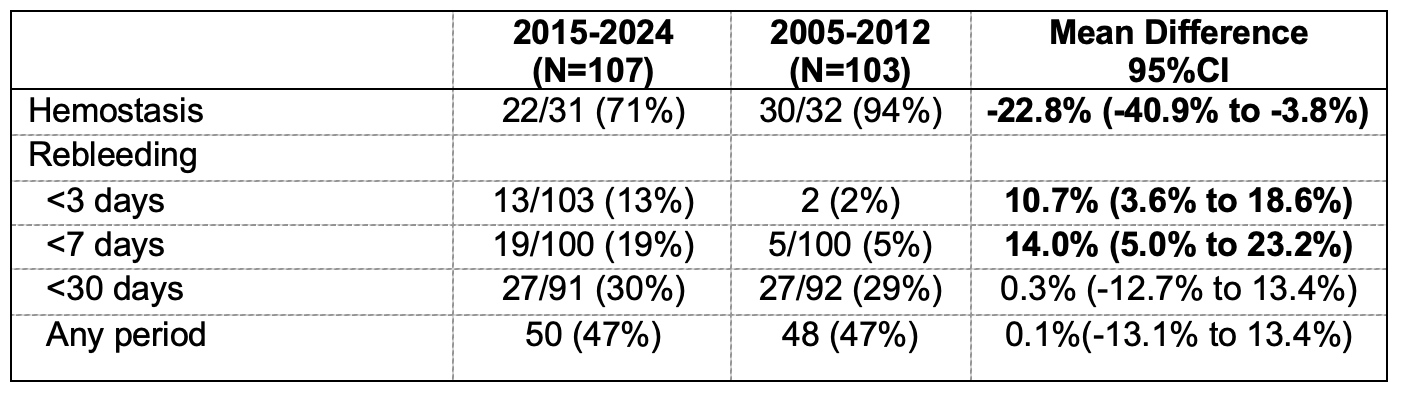
Figure: Table 1. Outcomes of 107 patients with upper GI bleeding from tumors
* Hemostasis was assessed only in patients with active bleeding at index endoscopy.
**Denominators for outcomes reflect the number of patients with information available at that time point.
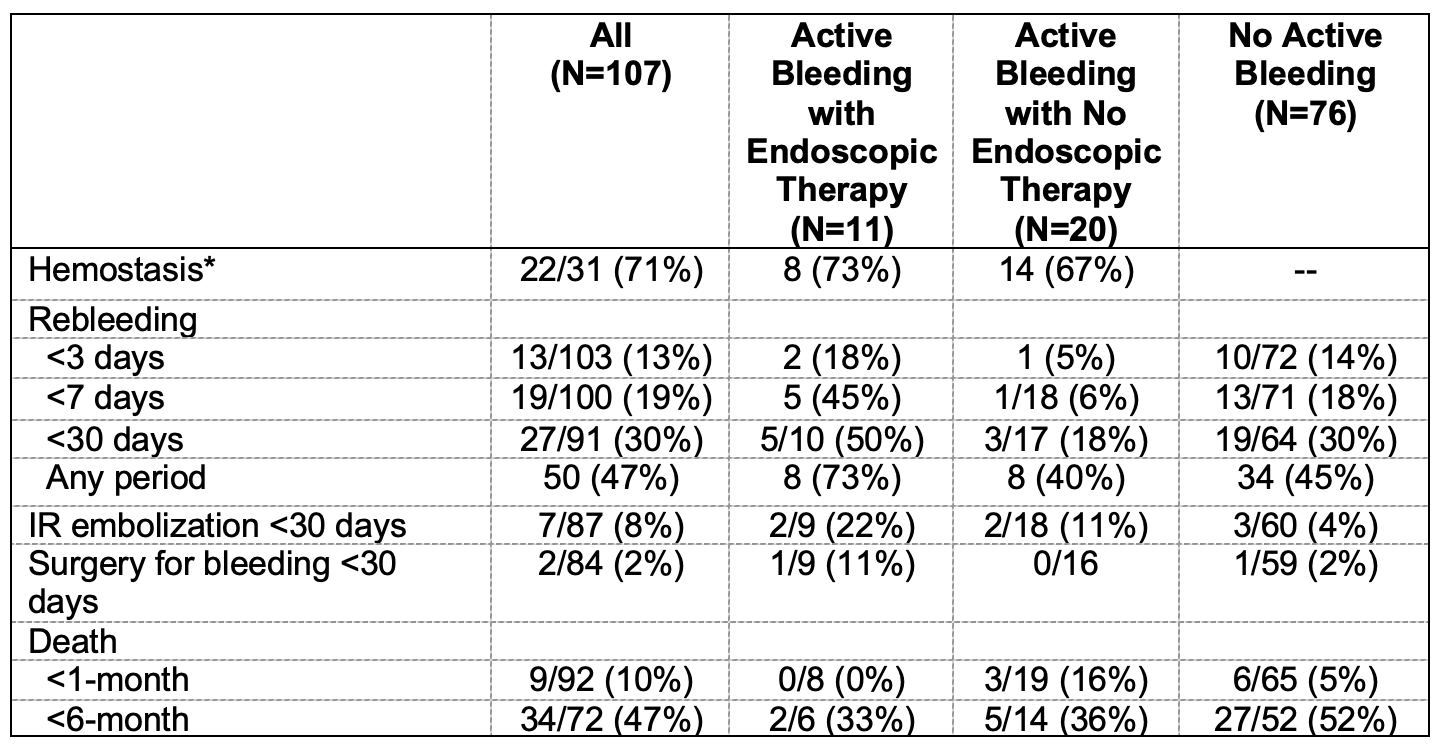
Figure: Table 2. Comparison in outcomes of patients with upper GI bleeding from tumors between 2015-2024 and 2005-2012
Disclosures:
Sean Dewberry indicated no relevant financial relationships.
Gevork Salmastyan indicated no relevant financial relationships.
Sarah Sheibani: Johnson and Johnson – Speakers Bureau.
Kathy Lui indicated no relevant financial relationships.
Loren Laine indicated no relevant financial relationships.
John Kim indicated no relevant financial relationships.
Sean Dewberry, MD1, Gevork Salmastyan, MD1, Sarah Sheibani, MD1, Kathy Lui, MD1, Loren Laine, MD2, John J.. Kim, MD, MS, FACG3. P0912 - Natural History and Outcomes of Patients With Upper GI Bleeding from Tumors, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Keck School of Medicine of the University of Southern California, Los Angeles, CA; 2Yale Digestive Diseases, New Haven, CT; 3Los Angeles General Medical Center, Los Angeles, CA
Introduction: Recent information evaluating the clinical course and outcomes in patients with upper GI bleeding from tumors outside clinical trials of endoscopic therapy is limited. We evaluated the outcomes of hospitalized patients with tumor-related upper GI bleeding receiving endoscopy at an urban US hospital and compared them to outcomes at the same center 10 years ago.
Methods: Consecutive patients hospitalized (6/2015-11/2024) with hematemesis, melena, and/or hematochezia receiving endoscopy were searched using ICD 9/10 codes for upper GI tumors. Procedure reports were reviewed to confirm biopsy-proven tumors without other causes of GI bleeding. Study endpoints included hemostasis at the conclusion of endoscopy and rebleeding. Rebleeding was defined as recurrent bleeding in patients without active bleeding on index endoscopy or whose bleeding stopped spontaneously or with endoscopic, interventional radiologic, or surgical intervention.
Results: 107 (mean age 58+13 years, 81 (76%) males) patients met study criteria with tumor originating from the esophagus in 13 (12%), stomach in 84 (79%), duodenum in 9 (8%), and jejunum in 1 (1%). At presentation, 62 (58%) patients did not have a prior diagnosis of malignancy and 66 (62%) had evidence of metastatic disease. Hemodynamic instability was observed in 50 (47%) patients, and 28 (26%) were on anti-thrombotic therapy. High-risk stigmata of bleeding were reported in 48 (45%) patients: active bleeding in 31 (29%) including spurting 2 (2%) and oozing 29 (27%), non-bleeding visible vessel in 3 (3%), and adherent clot in 14 (13%). Endoscopic therapy was performed in 19 (18%), including TC-325 hemostatic powder spray in 6 (6%), epinephrine injection alone in 6 (6%), hemoclips in 3 (3%), bipolar coagulation in 2 (2%), polysaccharide hemostatic powder in 1 (1%), and alcohol injection in 1 (1%). Overall, 9 (8%) patients did not attain hemostasis; 13 (13%) of 103 and 19 (19%) of 100 developed rebleeding within 3 and 7 days, respectively (Table 1). During a mean follow-up of 10.6+16.7 months, 50 (47%) patients developed rebleeding, similar to 48 (47%) of 103 from the 2005-2012 cohort (Table 2).
Discussion: Active bleeding was encountered in nearly one-third of patients with tumor-related upper GI bleeding. Most patients attained endoscopic hemostasis with or without therapy. Similar to a decade ago, initial control of bleeding was successful in most patients, but nearly a half developed long-term recurrent bleeding.

Figure: Table 1. Outcomes of 107 patients with upper GI bleeding from tumors
* Hemostasis was assessed only in patients with active bleeding at index endoscopy.
**Denominators for outcomes reflect the number of patients with information available at that time point.

Figure: Table 2. Comparison in outcomes of patients with upper GI bleeding from tumors between 2015-2024 and 2005-2012
Disclosures:
Sean Dewberry indicated no relevant financial relationships.
Gevork Salmastyan indicated no relevant financial relationships.
Sarah Sheibani: Johnson and Johnson – Speakers Bureau.
Kathy Lui indicated no relevant financial relationships.
Loren Laine indicated no relevant financial relationships.
John Kim indicated no relevant financial relationships.
Sean Dewberry, MD1, Gevork Salmastyan, MD1, Sarah Sheibani, MD1, Kathy Lui, MD1, Loren Laine, MD2, John J.. Kim, MD, MS, FACG3. P0912 - Natural History and Outcomes of Patients With Upper GI Bleeding from Tumors, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
