Sunday Poster Session
Category: GI Bleeding
P0910 - Adverse Events Associated With Esophageal Balloon Tamponade Devices: A 15-Year Retrospective Analysis of the FDA MAUDE Database
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Mohsin Chundrigar, MBBS (he/him/his)
Albany Medical Center
Albany, NY
Presenting Author(s)
Mohsin Chundrigar, MBBS, Osama Alshakhatreh, MD, Sarim Ansari, MBBS, Shahreen Ansari, MBBS, Khaled Elsokary, DO
Albany Medical Center, Albany, NY
Introduction: Esophageal balloon tamponade devices are critical interventions for controlling life-threatening variceal bleeding. Despite their therapeutic importance, limited data exists regarding device-related adverse events in post-marketing surveillance. This study analyzes the FDA MAUDE database to characterize adverse events associated with esophageal balloon tamponade devices.
Methods: A retrospective analysis of MAUDE database reports from 2010-2025 involving esophageal balloon tamponade devices was conducted. Reports were manually reviewed and categorized into device-related and patient-related adverse events. Duplicate reports were excluded. Event frequencies and percentages were calculated.
Results: A total of 40 valid reports were identified involving esophageal balloon tamponade devices. There were 54 device-related events and 52 patient-related events identified. Device-related events were predominantly material integrity issues (18.5%), inflation/deflation problems (16.7%), and deployment/removal difficulties (14.8%). Separation/detachment issues and burst/rupture events each occurred in 6 events (11.1%).
Patient-related events included problems with no impact or consequence (73.1%) and unavailable code (11.5%). Serious adverse events included death (3.8%), exsanguination (1.9%), and hemorrhage/bleeding (1.9%). Death was noted in 2 reports with an overall mortality rate of 5.0%.
Discussion: This analysis reveals important safety considerations for esophageal balloon tamponade devices, with device malfunction being predominant (85.0%). Material integrity issues (18.5%) and inflation/deflation problems (16.7%) were the most frequent device problems, highlighting concerns about structural durability and pressure management. Most patient-related events (73.1%) resulted in no clinical impact. However, serious adverse events including death (3.8%) emphasize the critical nature of proper device function in emergency scenarios. These findings highlight the need for improved device reliability and practitioner training.
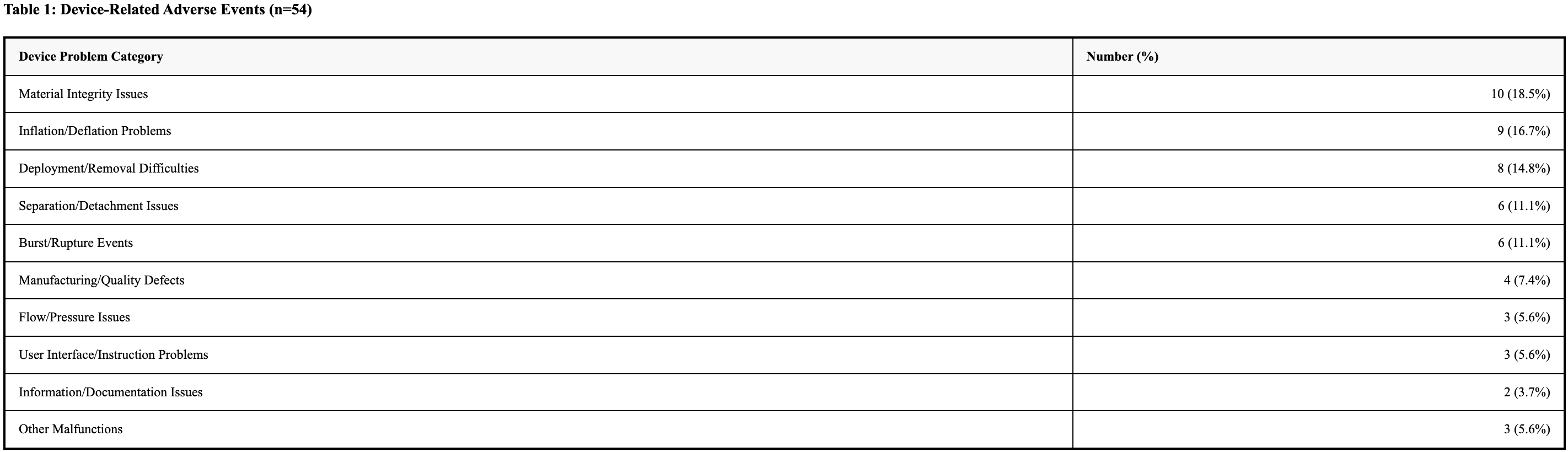
Figure: Table 1: Device-Related Adverse Events
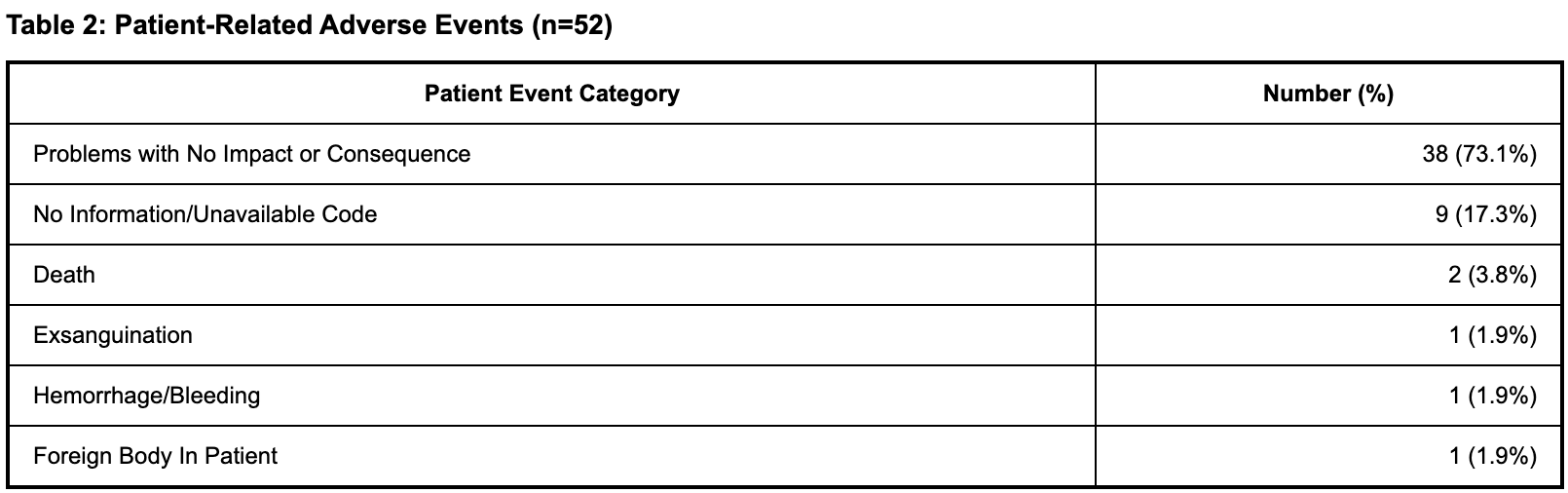
Figure: Table 2: Patient-Related Adverse Events
Disclosures:
Mohsin Chundrigar indicated no relevant financial relationships.
Osama Alshakhatreh indicated no relevant financial relationships.
Sarim Ansari indicated no relevant financial relationships.
Shahreen Ansari indicated no relevant financial relationships.
Khaled Elsokary indicated no relevant financial relationships.
Mohsin Chundrigar, MBBS, Osama Alshakhatreh, MD, Sarim Ansari, MBBS, Shahreen Ansari, MBBS, Khaled Elsokary, DO. P0910 - Adverse Events Associated With Esophageal Balloon Tamponade Devices: A 15-Year Retrospective Analysis of the FDA MAUDE Database, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Albany Medical Center, Albany, NY
Introduction: Esophageal balloon tamponade devices are critical interventions for controlling life-threatening variceal bleeding. Despite their therapeutic importance, limited data exists regarding device-related adverse events in post-marketing surveillance. This study analyzes the FDA MAUDE database to characterize adverse events associated with esophageal balloon tamponade devices.
Methods: A retrospective analysis of MAUDE database reports from 2010-2025 involving esophageal balloon tamponade devices was conducted. Reports were manually reviewed and categorized into device-related and patient-related adverse events. Duplicate reports were excluded. Event frequencies and percentages were calculated.
Results: A total of 40 valid reports were identified involving esophageal balloon tamponade devices. There were 54 device-related events and 52 patient-related events identified. Device-related events were predominantly material integrity issues (18.5%), inflation/deflation problems (16.7%), and deployment/removal difficulties (14.8%). Separation/detachment issues and burst/rupture events each occurred in 6 events (11.1%).
Patient-related events included problems with no impact or consequence (73.1%) and unavailable code (11.5%). Serious adverse events included death (3.8%), exsanguination (1.9%), and hemorrhage/bleeding (1.9%). Death was noted in 2 reports with an overall mortality rate of 5.0%.
Discussion: This analysis reveals important safety considerations for esophageal balloon tamponade devices, with device malfunction being predominant (85.0%). Material integrity issues (18.5%) and inflation/deflation problems (16.7%) were the most frequent device problems, highlighting concerns about structural durability and pressure management. Most patient-related events (73.1%) resulted in no clinical impact. However, serious adverse events including death (3.8%) emphasize the critical nature of proper device function in emergency scenarios. These findings highlight the need for improved device reliability and practitioner training.

Figure: Table 1: Device-Related Adverse Events

Figure: Table 2: Patient-Related Adverse Events
Disclosures:
Mohsin Chundrigar indicated no relevant financial relationships.
Osama Alshakhatreh indicated no relevant financial relationships.
Sarim Ansari indicated no relevant financial relationships.
Shahreen Ansari indicated no relevant financial relationships.
Khaled Elsokary indicated no relevant financial relationships.
Mohsin Chundrigar, MBBS, Osama Alshakhatreh, MD, Sarim Ansari, MBBS, Shahreen Ansari, MBBS, Khaled Elsokary, DO. P0910 - Adverse Events Associated With Esophageal Balloon Tamponade Devices: A 15-Year Retrospective Analysis of the FDA MAUDE Database, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

