Sunday Poster Session
Category: GI Bleeding
P0907 - Proton-Pump Inhibitors as Primary Prophylaxis for Corticosteroid Use: A Systematic Review and Meta-Analysis
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Narisara Tribuddharat, MD (she/her/hers)
St. Elizabeth's Medical Center, Boston University School of Medicine
Brighton, MA
Presenting Author(s)
Narisara Tribuddharat, MD1, Dimo Dimitrov, MD1, Angela Achkar, MD1, Ali Emre Bardak, MD2, Eli Morse, MD1, Koravich Lorlowhakarn, MD1, Sandeep Krishnan, MBBS, PhD2
1St. Elizabeth's Medical Center, Boston University School of Medicine, Brighton, MA; 2St. Elizabeth's Medical Center, Boston University School of Medicine, Boston, MA
Introduction: Corticosteroid use increases the risk of peptic ulcers and upper gastrointestinal bleeding (UGIB). Proton-pump inhibitors (PPIs) are commonly prescribed to manage these complications and are often used prophylactically for patients on corticosteroids. However, evidence supporting this remains limited. Systematic reviews are necessary to provide robust data regarding the efficacy of PPIs as primary prophylaxis in preventing UGIB compared to no PPI.
Methods: We did a comprehensive literature search in PubMed and Embase from inception to April 2025. We selected randomized-controlled trials (RCTs), cohort studies, or case-control studies that studied the rate of UGIB in patients using corticosteroids between those with PPIs primary prophylaxis versus no PPI prophylaxis. Other interventions, such as histamine-2 receptor antagonists, are included in the non-PPI group. The outcome of interest was UGIB rate. We conducted this meta-analysis and reported the pooled odds ratio (OR) with 95% confidence intervals (CI) using a random-effects model.
Results: A total of four studies met the inclusion criteria, including one RCT, one cohort study, and two case-control studies. The PPI dosages that were reported were pantoprazole 40 mg daily and omeprazole 20-40 mg daily with varying follow-up durations from one to six months. The durations of the PPI given were not specific, but either stated or implied from data that the patients were given PPI throughout the corticosteroid treatment or longer. The final analysis comprised a total of 4,334 patients (1,264 in the PPI group and 3,070 in the non-PPI group). Patients with PPI primary prophylaxis had a lower risk of UGIB than those without PPIs (OR, 0.66 [95% CI, 0.50, 0.87], p = 0.003).
Discussion: Our study showed that primary prophylaxis with PPIs significantly reduced the risk of UGIB in patients using corticosteroids.
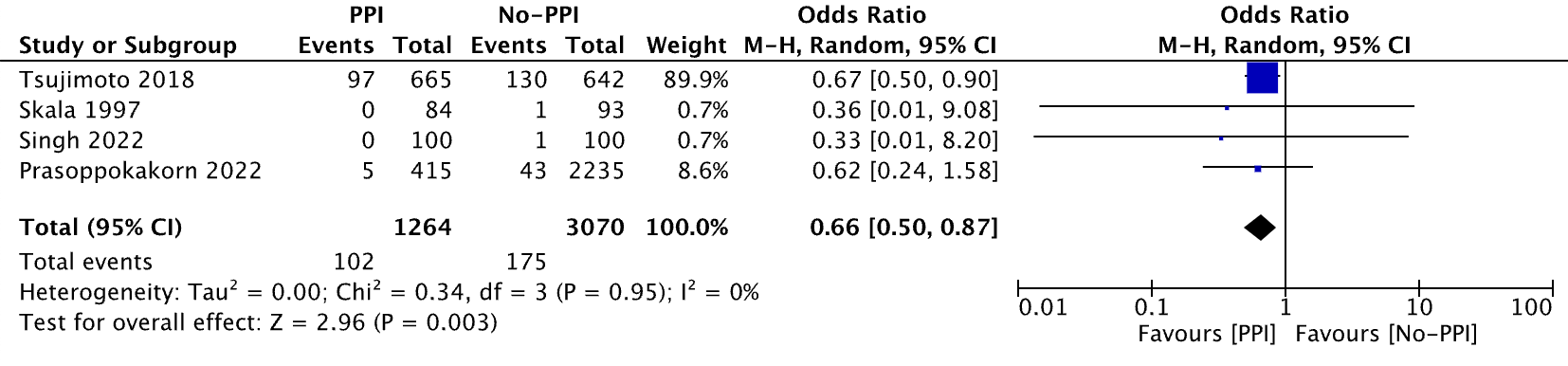
Figure: Fig 1. Rate of UGIB in PPI group compared to non-PPI group
Disclosures:
Narisara Tribuddharat indicated no relevant financial relationships.
Dimo Dimitrov indicated no relevant financial relationships.
Angela Achkar indicated no relevant financial relationships.
Ali Emre Bardak indicated no relevant financial relationships.
Eli Morse indicated no relevant financial relationships.
Koravich Lorlowhakarn indicated no relevant financial relationships.
Sandeep Krishnan indicated no relevant financial relationships.
Narisara Tribuddharat, MD1, Dimo Dimitrov, MD1, Angela Achkar, MD1, Ali Emre Bardak, MD2, Eli Morse, MD1, Koravich Lorlowhakarn, MD1, Sandeep Krishnan, MBBS, PhD2. P0907 - Proton-Pump Inhibitors as Primary Prophylaxis for Corticosteroid Use: A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1St. Elizabeth's Medical Center, Boston University School of Medicine, Brighton, MA; 2St. Elizabeth's Medical Center, Boston University School of Medicine, Boston, MA
Introduction: Corticosteroid use increases the risk of peptic ulcers and upper gastrointestinal bleeding (UGIB). Proton-pump inhibitors (PPIs) are commonly prescribed to manage these complications and are often used prophylactically for patients on corticosteroids. However, evidence supporting this remains limited. Systematic reviews are necessary to provide robust data regarding the efficacy of PPIs as primary prophylaxis in preventing UGIB compared to no PPI.
Methods: We did a comprehensive literature search in PubMed and Embase from inception to April 2025. We selected randomized-controlled trials (RCTs), cohort studies, or case-control studies that studied the rate of UGIB in patients using corticosteroids between those with PPIs primary prophylaxis versus no PPI prophylaxis. Other interventions, such as histamine-2 receptor antagonists, are included in the non-PPI group. The outcome of interest was UGIB rate. We conducted this meta-analysis and reported the pooled odds ratio (OR) with 95% confidence intervals (CI) using a random-effects model.
Results: A total of four studies met the inclusion criteria, including one RCT, one cohort study, and two case-control studies. The PPI dosages that were reported were pantoprazole 40 mg daily and omeprazole 20-40 mg daily with varying follow-up durations from one to six months. The durations of the PPI given were not specific, but either stated or implied from data that the patients were given PPI throughout the corticosteroid treatment or longer. The final analysis comprised a total of 4,334 patients (1,264 in the PPI group and 3,070 in the non-PPI group). Patients with PPI primary prophylaxis had a lower risk of UGIB than those without PPIs (OR, 0.66 [95% CI, 0.50, 0.87], p = 0.003).
Discussion: Our study showed that primary prophylaxis with PPIs significantly reduced the risk of UGIB in patients using corticosteroids.

Figure: Fig 1. Rate of UGIB in PPI group compared to non-PPI group
Disclosures:
Narisara Tribuddharat indicated no relevant financial relationships.
Dimo Dimitrov indicated no relevant financial relationships.
Angela Achkar indicated no relevant financial relationships.
Ali Emre Bardak indicated no relevant financial relationships.
Eli Morse indicated no relevant financial relationships.
Koravich Lorlowhakarn indicated no relevant financial relationships.
Sandeep Krishnan indicated no relevant financial relationships.
Narisara Tribuddharat, MD1, Dimo Dimitrov, MD1, Angela Achkar, MD1, Ali Emre Bardak, MD2, Eli Morse, MD1, Koravich Lorlowhakarn, MD1, Sandeep Krishnan, MBBS, PhD2. P0907 - Proton-Pump Inhibitors as Primary Prophylaxis for Corticosteroid Use: A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
