Sunday Poster Session
Category: IBD
P1050 - Second TNFi Therapy Versus Alternative Biologic Therapy in Patients With Inflammatory Bowel Disease and Previous TNFi Exposure and the Risk of Incident Immune-Mediated Inflammatory Diseases: A Real-World Cohort Study
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Himsikhar Khataniar, MD (he/him/his)
Allegheny General Hospital
Pittsburgh, PA
Presenting Author(s)
Aakash Desai, MD1, Himsikhar Khataniar, MD2, Jana G. Hashash, MD, MSc, FACG3, Gursimran Kochhar, MD1, Francis A.. Farraye, MD, MSc, MACG3
1Allegheny Health Network, Pittsburgh, PA; 2Allegheny General Hospital, Pittsburgh, PA; 3Mayo Clinic, Jacksonville, FL
Introduction: Immune-mediated inflammatory diseases (IMIDs) such as psoriasis and rheumatoid arthritis can develop during tumor-necrosis-factor inhibitor (TNFi) therapy for inflammatory bowel disease (IBD). The impact of exposure to a second TNFi or switching to a biologic therapy with a different mechanism of action on the risk of IMIDs is unknown.
Methods: Using the TriNetX US Collaborative Network (2014-2023), we identified adults with Crohn’s disease (CD) or ulcerative colitis (UC) with previous exposure to infliximab or adalimumab who were either switched to a second TNFi or ustekinumab/vedolizumab. Patients with any pre-existing IMID prior to switching to a second biologic were excluded. The primary outcome was the risk of IMID in the TNFi cohort compared to ustekinumab/vedolizumab cohort (control cohort) within 5 years. A 1:1 propensity score matching (PSM) was performed for demographics, IBD type, CD phenotype, nicotine dependence, previous surgery, steroid use and immunomodulator use. Cox proportional hazard model was used to identify risk factors for new onset IMID in the TNFi cohort.
Results: Among 14,770 patients, 6129 (41.5%) received a second TNFi (mean age 34.4 +/- 16.7, 49.6% female, 73.9% White, 80% CD) and 8641 (58.5%) were switched to ustekinumab or vedolizumab (mean age 39.7 +/- 17, 51.3% female, 75.3% White, 77.3% CD). Mean follow-up after the switch was 1354 and 1316 days, respectively. After PSM, the TNFi cohort had a higher risk of IMID compared to the control cohort (10.8% vs 6.9%, adjusted hazard ratio [aHR] 1.57, 95 % CI 1.37–1.79; log rank p< 0.0001). The increased risk was seen in both UC (aHR 1.90, 95% CI 1.53-2.37) and CD (aHR 1.43, 95% CI 1.22-1.67). Sensitivity analysis after excluding psoriasis, rheumatoid arthritis and ankylosing spondylitis also showed an increased risk of IMID in the TNFi cohort (aHR 1.67, 95% CI 1.37–2.05). Sub-group analysis based on age and sex also showed an increased risk of IMID in the TNFi cohort. Within the TNFi cohort, age ≥40 years, primary sclerosing cholangitis and methotrexate use predicted IMID, whereas male sex and concomitant azathioprine were protective.
Discussion: In this large real-world IBD cohort with exposure to a TNFi, second TNFi use was associated with a higher risk of de-novo IMIDs compared to switch to ustekinumab or vedolizumab.
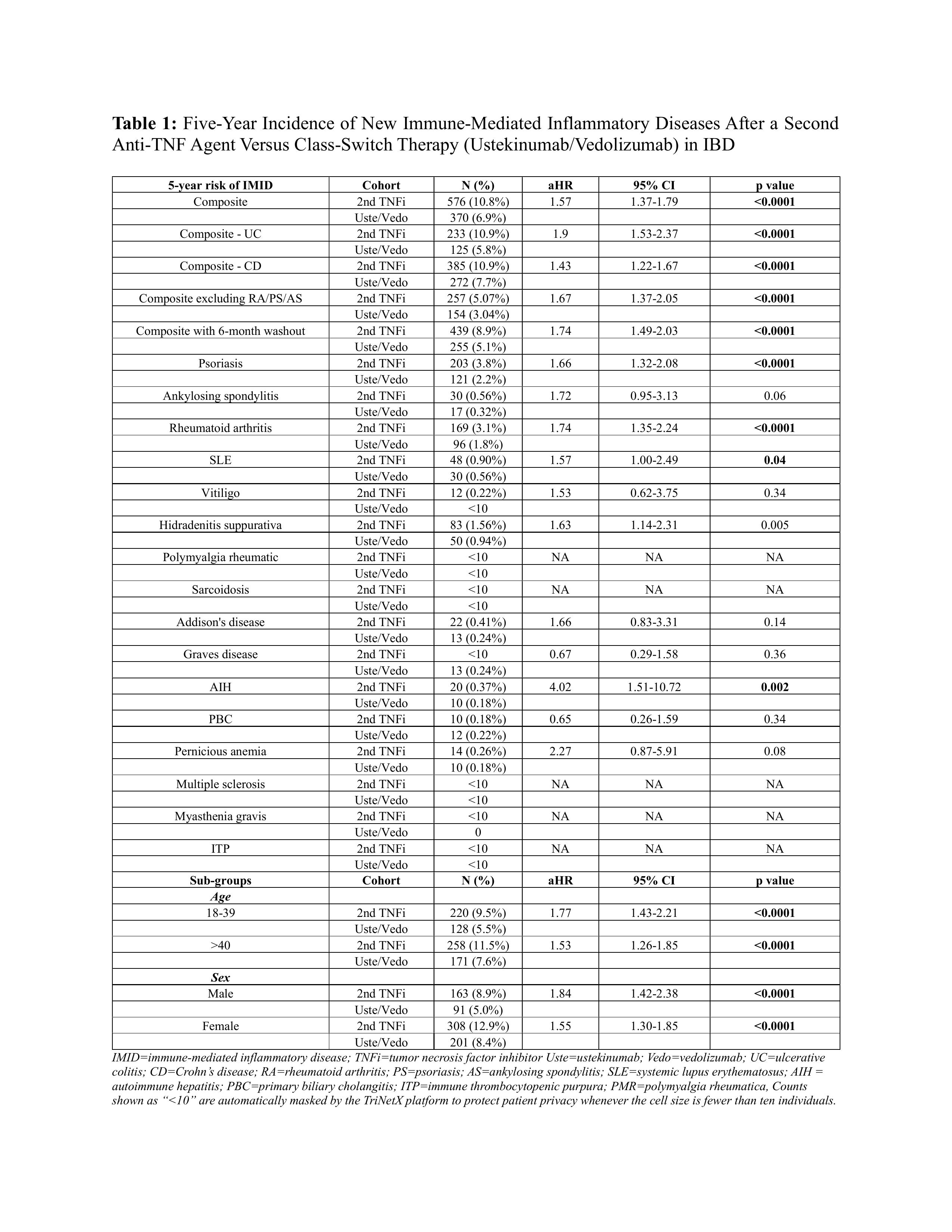
Figure: Table 1: Five-Year Incidence of New Immune-Mediated Inflammatory Diseases After a Second Anti-TNF Agent Versus Class-Switch Therapy (Ustekinumab/Vedolizumab) in IBD
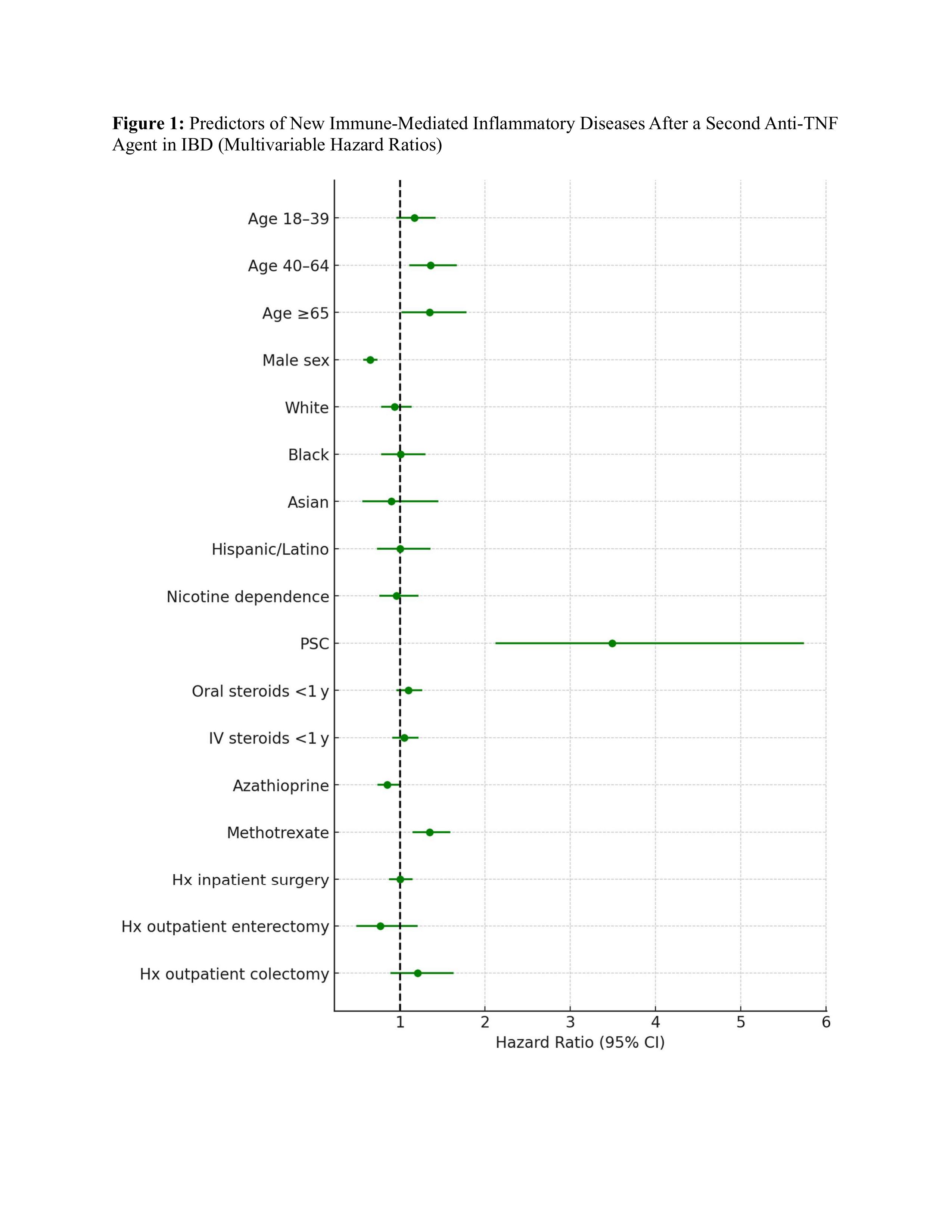
Figure: Figure 1: Predictors of New Immune-Mediated Inflammatory Diseases After a Second Anti-TNF Agent in IBD (Multivariable Hazard Ratios)
Disclosures:
Aakash Desai indicated no relevant financial relationships.
Himsikhar Khataniar indicated no relevant financial relationships.
Jana Hashash: BMS – Ad Board.
Gursimran Kochhar indicated no relevant financial relationships.
Francis Farraye: Astellas – Advisory Committee/Board Member. Avalo – Advisory Committee/Board Member. Bausch – Advisory Committee/Board Member. BMS – Advisory Committee/Board Member. Braintree Labs – Advisory Committee/Board Member. Fresenius Kabi – Advisory Committee/Board Member. GI Reviewers – Independent Contractor. IBD Educational Group – Independent Contractor. Iterative Health – Advisory Committee/Board Member, Stock Options. Janssen – Advisory Committee/Board Member. Lilly – DSMB. Pfizer – Advisory Committee/Board Member. Pharmacosmos – Advisory Committee/Board Member. Sandoz – Advisory Committee/Board Member. Viatris – Advisory Committee/Board Member.
Aakash Desai, MD1, Himsikhar Khataniar, MD2, Jana G. Hashash, MD, MSc, FACG3, Gursimran Kochhar, MD1, Francis A.. Farraye, MD, MSc, MACG3. P1050 - Second TNFi Therapy Versus Alternative Biologic Therapy in Patients With Inflammatory Bowel Disease and Previous TNFi Exposure and the Risk of Incident Immune-Mediated Inflammatory Diseases: A Real-World Cohort Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Allegheny Health Network, Pittsburgh, PA; 2Allegheny General Hospital, Pittsburgh, PA; 3Mayo Clinic, Jacksonville, FL
Introduction: Immune-mediated inflammatory diseases (IMIDs) such as psoriasis and rheumatoid arthritis can develop during tumor-necrosis-factor inhibitor (TNFi) therapy for inflammatory bowel disease (IBD). The impact of exposure to a second TNFi or switching to a biologic therapy with a different mechanism of action on the risk of IMIDs is unknown.
Methods: Using the TriNetX US Collaborative Network (2014-2023), we identified adults with Crohn’s disease (CD) or ulcerative colitis (UC) with previous exposure to infliximab or adalimumab who were either switched to a second TNFi or ustekinumab/vedolizumab. Patients with any pre-existing IMID prior to switching to a second biologic were excluded. The primary outcome was the risk of IMID in the TNFi cohort compared to ustekinumab/vedolizumab cohort (control cohort) within 5 years. A 1:1 propensity score matching (PSM) was performed for demographics, IBD type, CD phenotype, nicotine dependence, previous surgery, steroid use and immunomodulator use. Cox proportional hazard model was used to identify risk factors for new onset IMID in the TNFi cohort.
Results: Among 14,770 patients, 6129 (41.5%) received a second TNFi (mean age 34.4 +/- 16.7, 49.6% female, 73.9% White, 80% CD) and 8641 (58.5%) were switched to ustekinumab or vedolizumab (mean age 39.7 +/- 17, 51.3% female, 75.3% White, 77.3% CD). Mean follow-up after the switch was 1354 and 1316 days, respectively. After PSM, the TNFi cohort had a higher risk of IMID compared to the control cohort (10.8% vs 6.9%, adjusted hazard ratio [aHR] 1.57, 95 % CI 1.37–1.79; log rank p< 0.0001). The increased risk was seen in both UC (aHR 1.90, 95% CI 1.53-2.37) and CD (aHR 1.43, 95% CI 1.22-1.67). Sensitivity analysis after excluding psoriasis, rheumatoid arthritis and ankylosing spondylitis also showed an increased risk of IMID in the TNFi cohort (aHR 1.67, 95% CI 1.37–2.05). Sub-group analysis based on age and sex also showed an increased risk of IMID in the TNFi cohort. Within the TNFi cohort, age ≥40 years, primary sclerosing cholangitis and methotrexate use predicted IMID, whereas male sex and concomitant azathioprine were protective.
Discussion: In this large real-world IBD cohort with exposure to a TNFi, second TNFi use was associated with a higher risk of de-novo IMIDs compared to switch to ustekinumab or vedolizumab.

Figure: Table 1: Five-Year Incidence of New Immune-Mediated Inflammatory Diseases After a Second Anti-TNF Agent Versus Class-Switch Therapy (Ustekinumab/Vedolizumab) in IBD

Figure: Figure 1: Predictors of New Immune-Mediated Inflammatory Diseases After a Second Anti-TNF Agent in IBD (Multivariable Hazard Ratios)
Disclosures:
Aakash Desai indicated no relevant financial relationships.
Himsikhar Khataniar indicated no relevant financial relationships.
Jana Hashash: BMS – Ad Board.
Gursimran Kochhar indicated no relevant financial relationships.
Francis Farraye: Astellas – Advisory Committee/Board Member. Avalo – Advisory Committee/Board Member. Bausch – Advisory Committee/Board Member. BMS – Advisory Committee/Board Member. Braintree Labs – Advisory Committee/Board Member. Fresenius Kabi – Advisory Committee/Board Member. GI Reviewers – Independent Contractor. IBD Educational Group – Independent Contractor. Iterative Health – Advisory Committee/Board Member, Stock Options. Janssen – Advisory Committee/Board Member. Lilly – DSMB. Pfizer – Advisory Committee/Board Member. Pharmacosmos – Advisory Committee/Board Member. Sandoz – Advisory Committee/Board Member. Viatris – Advisory Committee/Board Member.
Aakash Desai, MD1, Himsikhar Khataniar, MD2, Jana G. Hashash, MD, MSc, FACG3, Gursimran Kochhar, MD1, Francis A.. Farraye, MD, MSc, MACG3. P1050 - Second TNFi Therapy Versus Alternative Biologic Therapy in Patients With Inflammatory Bowel Disease and Previous TNFi Exposure and the Risk of Incident Immune-Mediated Inflammatory Diseases: A Real-World Cohort Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
