Sunday Poster Session
Category: IBD
P1045 - Adverse Outcomes in Older IBD Patients: No Significant Safety Differences Across Therapy Classes
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Neal K. Bhachawat, DO
Houston Methodist Hospital
Houston, TX
Presenting Author(s)
Neal K. Bhachawat, DO1, Oscar Noble, MD2, Wahibah Hannan, MD1, Megan Lewis, DO1, Poojasree Pasupuleti, MS2, Fadi Shehadeh, MSc1, Bincy Abraham, MD, MS, FACG3, Malcolm Irani, DO1, Kerri Glassner, DO1, Christopher Fan, MD4
1Houston Methodist Hospital, Houston, TX; 2Houston Methodist Hospital, Division of Gastroenterology and Hepatology, Lynda K. and David M. Underwood Center for Digestive Health / Houston Methodist Research Institute, Department of Medicine, Houston, TX; 3Division of Gastroenterology & Hepatology, Houston Methodist-Weill Cornell, Houston, TX; 4Houston Methodist Hospital, Division of Gastroenterology and Hepatology, Lynda K. and David M. Underwood Center for Digestive Health / Houston Methodist Research Institute, Department of Medicine / Weill Cornell Medical College, Cornell University, Houston, TX
Introduction: Although inflammatory bowel disease (IBD) is increasingly prevalent in older adults, this population remains underrepresented in studies evaluating the safety of advanced therapies. This study aims to characterize adverse outcomes associated with advanced IBD therapies in patients aged ≥60 years.
Methods: Patients aged ≥60 years with ICD-10 codes for Crohn’s disease (CD) or ulcerative colitis (UC) treated with advanced IBD therapies were identified from the electronic medical record. Advanced therapies included anti-TNF-α agents, anti-interleukin-12/23 agents, janus kinase inhibitors, and anti-integrins; immunomodulators were incorporated as adjunct therapy in select patients. Primary outcome was a composite of drug complications, infection, and malignancy and a secondary outcome looked at hospitalization, surgery or death. Outcomes were validated through manual chart review. A univariate analysis compared patients with and without adverse outcomes. A multivariate Cox proportional hazard models evaluated time-to-event from therapy initiation to outcomes.
Results: A total of 267 patients were included; 32% experienced at least one adverse outcome. Baseline demographics and clinical characteristics were similar between groups (Table 1). Anti-TNF therapy was associated with increased risk and anti-IL12/23 with reduced risk in univariate analysis, but neither remained significant in multivariate models. In the Cox model evaluating the primary composite outcome of drug complications, infections, and malignancy, significant predictors were BMI < 18.5 kg/m² (HR 3.84, p=0.022) and severe Charlson comorbidity index (CCI) burden (HR 2.88, p=0.019). For hospitalization, surgery, or death, older age at treatment initiation (HR 0.93, p=0.020) and a UC diagnosis (vs. CD; HR 0.35, p=0.034) were protective, whereas moderate CCI burden (HR 2.96, p=0.025) significantly increased risk. No drug class was shown to independently predict adverse outcomes.
Discussion: In this cohort of IBD patients ≥60 years, no advanced therapy class was associated with increased risk of adverse outcomes. Significant predictors of adverse outcomes were low BMI in the drug complications, infection, and malignancy group, while a higher comorbidity burden category was a predictor in both composite outcome groups. Conversely, older age at treatment initiation and having UC were protective against hospitalization, surgery, and death. These findings support the safe and individualized use of advanced therapies in older IBD patients.
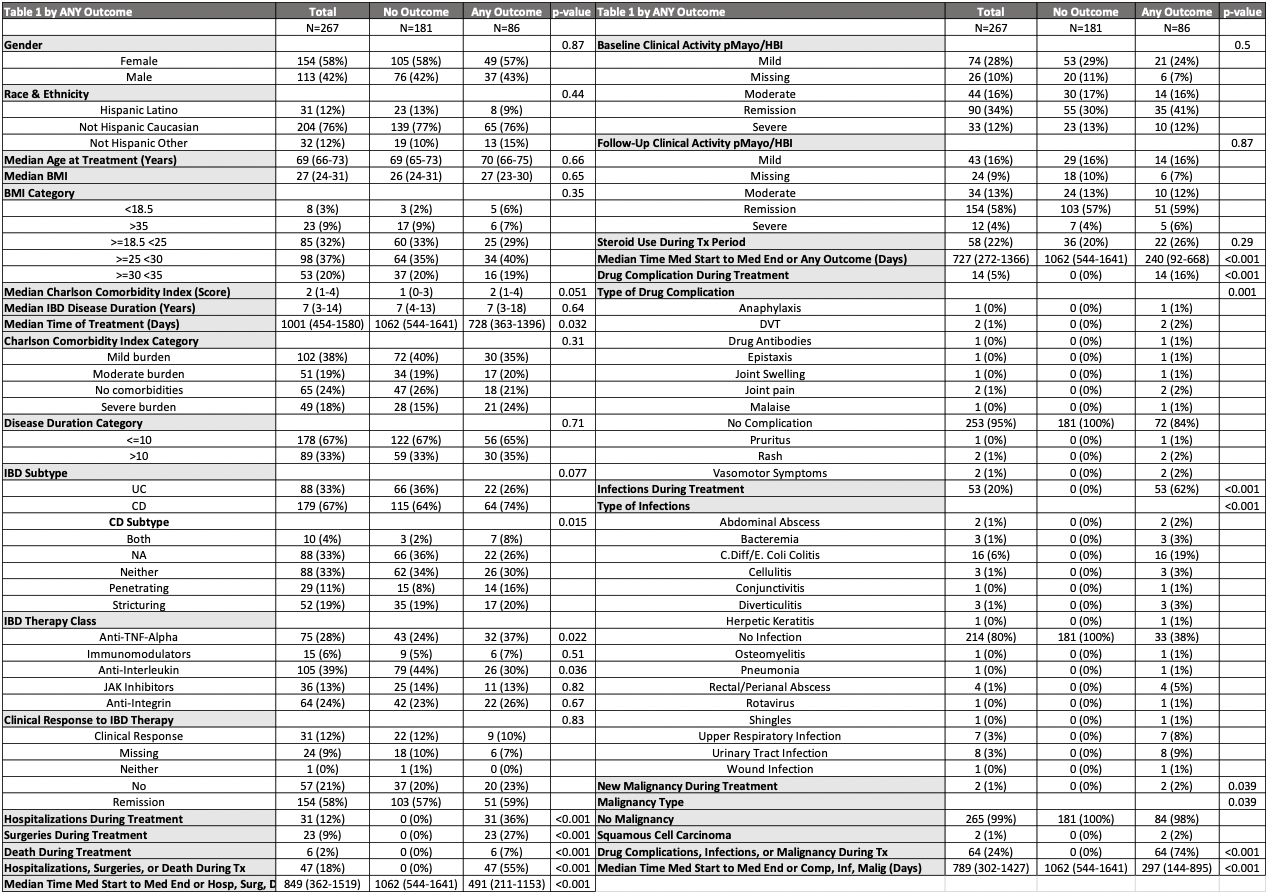
Figure: Table 1: Characteristics of the cohort by any outcome with univariate analysis.
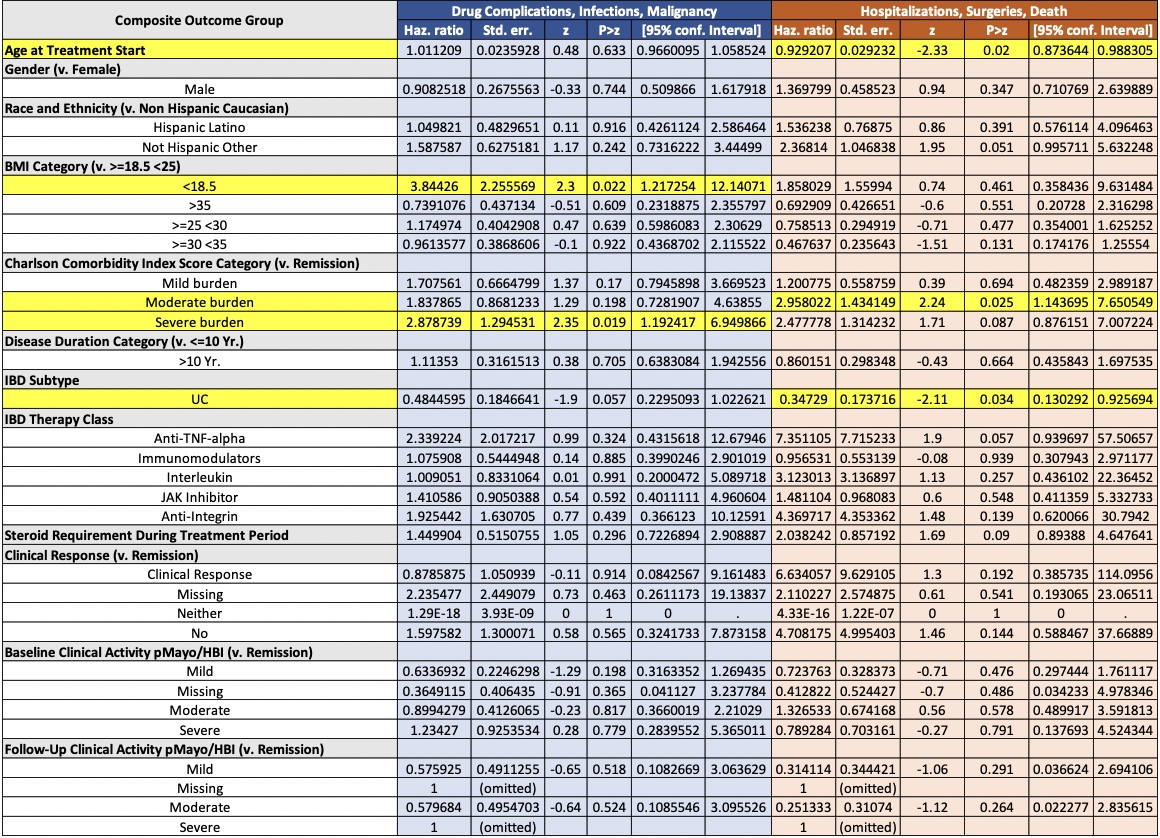
Figure: Table 2: COX-proportional hazards model for the composite outcome of drug complications, infections, and malignancy (blue). COX-proportional hazards model for the composite outcome of hospitalizations, surgeries, and death (orange). p-values <0.05 are highlighted in yellow.
Disclosures:
Neal Bhachawat indicated no relevant financial relationships.
Oscar Noble indicated no relevant financial relationships.
Wahibah Hannan indicated no relevant financial relationships.
Megan Lewis indicated no relevant financial relationships.
Poojasree Pasupuleti indicated no relevant financial relationships.
Fadi Shehadeh indicated no relevant financial relationships.
Bincy Abraham: Abbvie – Consultant, Speakers Bureau. Celltrion – Consultant. Eli Lilly – Consultant, Speakers Bureau. Johnson and Johnson – Consultant, Speakers Bureau. Pfizer – Consultant. Sanofi – Consultant. Takeda – Consultant, Speakers Bureau.
Malcolm Irani: ABBVIE – Speakers Bureau.
Kerri Glassner indicated no relevant financial relationships.
Christopher Fan indicated no relevant financial relationships.
Neal K. Bhachawat, DO1, Oscar Noble, MD2, Wahibah Hannan, MD1, Megan Lewis, DO1, Poojasree Pasupuleti, MS2, Fadi Shehadeh, MSc1, Bincy Abraham, MD, MS, FACG3, Malcolm Irani, DO1, Kerri Glassner, DO1, Christopher Fan, MD4. P1045 - Adverse Outcomes in Older IBD Patients: No Significant Safety Differences Across Therapy Classes, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Houston Methodist Hospital, Houston, TX; 2Houston Methodist Hospital, Division of Gastroenterology and Hepatology, Lynda K. and David M. Underwood Center for Digestive Health / Houston Methodist Research Institute, Department of Medicine, Houston, TX; 3Division of Gastroenterology & Hepatology, Houston Methodist-Weill Cornell, Houston, TX; 4Houston Methodist Hospital, Division of Gastroenterology and Hepatology, Lynda K. and David M. Underwood Center for Digestive Health / Houston Methodist Research Institute, Department of Medicine / Weill Cornell Medical College, Cornell University, Houston, TX
Introduction: Although inflammatory bowel disease (IBD) is increasingly prevalent in older adults, this population remains underrepresented in studies evaluating the safety of advanced therapies. This study aims to characterize adverse outcomes associated with advanced IBD therapies in patients aged ≥60 years.
Methods: Patients aged ≥60 years with ICD-10 codes for Crohn’s disease (CD) or ulcerative colitis (UC) treated with advanced IBD therapies were identified from the electronic medical record. Advanced therapies included anti-TNF-α agents, anti-interleukin-12/23 agents, janus kinase inhibitors, and anti-integrins; immunomodulators were incorporated as adjunct therapy in select patients. Primary outcome was a composite of drug complications, infection, and malignancy and a secondary outcome looked at hospitalization, surgery or death. Outcomes were validated through manual chart review. A univariate analysis compared patients with and without adverse outcomes. A multivariate Cox proportional hazard models evaluated time-to-event from therapy initiation to outcomes.
Results: A total of 267 patients were included; 32% experienced at least one adverse outcome. Baseline demographics and clinical characteristics were similar between groups (Table 1). Anti-TNF therapy was associated with increased risk and anti-IL12/23 with reduced risk in univariate analysis, but neither remained significant in multivariate models. In the Cox model evaluating the primary composite outcome of drug complications, infections, and malignancy, significant predictors were BMI < 18.5 kg/m² (HR 3.84, p=0.022) and severe Charlson comorbidity index (CCI) burden (HR 2.88, p=0.019). For hospitalization, surgery, or death, older age at treatment initiation (HR 0.93, p=0.020) and a UC diagnosis (vs. CD; HR 0.35, p=0.034) were protective, whereas moderate CCI burden (HR 2.96, p=0.025) significantly increased risk. No drug class was shown to independently predict adverse outcomes.
Discussion: In this cohort of IBD patients ≥60 years, no advanced therapy class was associated with increased risk of adverse outcomes. Significant predictors of adverse outcomes were low BMI in the drug complications, infection, and malignancy group, while a higher comorbidity burden category was a predictor in both composite outcome groups. Conversely, older age at treatment initiation and having UC were protective against hospitalization, surgery, and death. These findings support the safe and individualized use of advanced therapies in older IBD patients.

Figure: Table 1: Characteristics of the cohort by any outcome with univariate analysis.

Figure: Table 2: COX-proportional hazards model for the composite outcome of drug complications, infections, and malignancy (blue). COX-proportional hazards model for the composite outcome of hospitalizations, surgeries, and death (orange). p-values <0.05 are highlighted in yellow.
Disclosures:
Neal Bhachawat indicated no relevant financial relationships.
Oscar Noble indicated no relevant financial relationships.
Wahibah Hannan indicated no relevant financial relationships.
Megan Lewis indicated no relevant financial relationships.
Poojasree Pasupuleti indicated no relevant financial relationships.
Fadi Shehadeh indicated no relevant financial relationships.
Bincy Abraham: Abbvie – Consultant, Speakers Bureau. Celltrion – Consultant. Eli Lilly – Consultant, Speakers Bureau. Johnson and Johnson – Consultant, Speakers Bureau. Pfizer – Consultant. Sanofi – Consultant. Takeda – Consultant, Speakers Bureau.
Malcolm Irani: ABBVIE – Speakers Bureau.
Kerri Glassner indicated no relevant financial relationships.
Christopher Fan indicated no relevant financial relationships.
Neal K. Bhachawat, DO1, Oscar Noble, MD2, Wahibah Hannan, MD1, Megan Lewis, DO1, Poojasree Pasupuleti, MS2, Fadi Shehadeh, MSc1, Bincy Abraham, MD, MS, FACG3, Malcolm Irani, DO1, Kerri Glassner, DO1, Christopher Fan, MD4. P1045 - Adverse Outcomes in Older IBD Patients: No Significant Safety Differences Across Therapy Classes, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
