Sunday Poster Session
Category: IBD
P1044 - Efficacy of Etrasimod by Disease Duration: An Analysis of the Phase 3 ELEVATE UC Clinical Program
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Edward L. Barnes, MD, MPH
Multidisciplinary Inflammatory Bowel Diseases Center, Division of Gastroenterology and Hepatology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
Chapel Hill, NC
Presenting Author(s)
Edward L. Barnes, MD, MPH1, Fernando Magro, MD, PhD2, Hiroshi Nakase, MD, PhD3, Raymond K. K. Cross, MD, MS, FACG4, Shannon Chang, MD5, Faten Aberra, MD, MSCE6, Jesse Green, MD7, Laure Kouyoudjian, MD, MS7, Abhishek Bhattacharjee, PhD8, Maria Kudela, PhD9, Martina Goetsch, MD10, Geert R. D'Haens, MD, PhD11
1Multidisciplinary Inflammatory Bowel Diseases Center, Division of Gastroenterology and Hepatology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA, Chapel Hill, NC; 2CINTESIS@RISE, Faculty of Medicine, University of Porto, 4200-450, Porto, Portugal, Porto, Porto, Portugal; 3Department of Gastroenterology and Hepatology, Sapporo Medical University School of Medicine, Hokkaido, Japan, Sapporo, Hokkaido, Japan; 4Division of Gastroenterology and Hepatology, Melissa L. Posner Institute for Digestive Health & Liver Disease at Mercy Medical Center, Baltimore, MD, USA, Baltimore, MD; 5New York University Langone Health, New York, NY, USA, New York, NY; 6Division of Gastroenterology, Hospital of the University of Pennsylvania, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA, Philadelphia, PA; 7Pfizer Inc, Collegeville, PA, USA, Collegeville, PA; 8Pfizer Healthcare India Private Ltd, Chennai, India, Chennai, Tamil Nadu, India; 9Pfizer Inc, Cambridge, MA, USA, Cambridge, MA; 10Pfizer AG, Zürich, Switzerland, Zürich, Zurich, Switzerland; 11Department of Gastroenterology and Hepatology, Amsterdam University Medical Centers, Amsterdam, The Netherlands, Amsterdam, Noord-Holland, Netherlands
Introduction: Ulcerative colitis (UC) can lead to irreversible damage, highlighting the importance of early intervention. While disease duration impacts response to therapy in Crohn’s disease,1,2 similar evidence in UC is lacking.3 Etrasimod is an oral, once-daily (QD), selective sphingosine 1-phosphate (S1P)1,4,5 receptor modulator for the treatment of moderately to severely active UC. We evaluated the efficacy of etrasimod 2 mg QD vs placebo within disease duration subgroups in the ELEVATE UC clinical program.
Methods: In this post hoc analysis of ELEVATE UC 52 (NCT03945188) and ELEVATE UC 12 (NCT03996369), patients were assigned by disease duration (calculated using the date of diagnosis) into three subgroups: ≤ 2 years, > 2 to ≤ 5 years, > 5 years. In each disease duration subgroup, treatment comparisons and 2-sided p</em> values (reported without adjustment to multiple comparisons) were obtained using the Cochran–Mantel–Haenszel method for clinical remission, clinical response, symptomatic remission, endoscopic improvement, and endoscopic improvement-histologic remission at Week 12 and Week 52, and corticosteroid-free and sustained clinical remission at Week 52.
Results: Baseline characteristics were generally balanced within each subgroup; however, a higher proportion of patients in the ≤ 2 years subgroup were biologic/Janus kinase inhibitor-naïve vs the other two subgroups (Table 1). At Week 12 in all subgroups, greater proportions of patients receiving etrasimod vs placebo met criteria for all endpoints (p < 0.05; Table 2). At Week 52, greater proportions of patients receiving etrasimod vs placebo met criteria for all endpoints in the > 5 years subgroup (p < 0.05), and met criteria for most endpoints in the ≤ 2 years and > 2 to ≤ 5 years subgroups (Table 2).
Discussion: Etrasimod demonstrated significant induction and maintenance efficacy vs placebo in both symptomatic and endoscopic endpoints, irrespective of disease duration. These results highlight the efficacy of etrasimod for patients with shorter or longer duration of UC. Future analyses are needed to compare efficacy across disease duration subgroups and longer-term maintenance of response.
Refs:
1. Ungaro R et al. Aliment Pharmacol Ther 2020; 51: 831–842.
2. Noor NM et al. Lancet Gastroenterol Hepatol 2024; 9: 415–427.
3. Solitano V et al. J Clin Med 2020; 9: 2646.
Pfizer’s generative artificial intelligence tool MAIA was used to assist production of the abstract first draft. Authors reviewed/edited and take responsibility for the content.
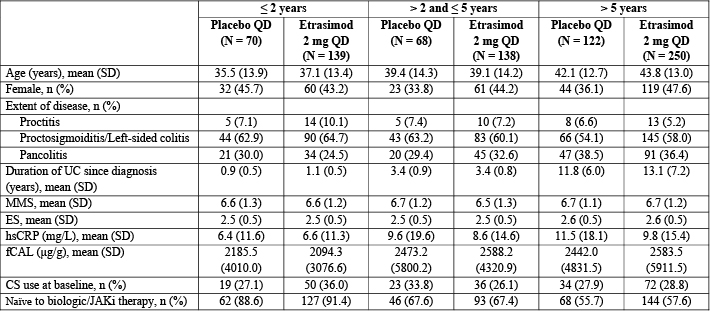
Figure: Table 1. Patient demographics and baseline characteristics by disease duration subgroup in the pooled ELEVATE UC 52 and ELEVATE UC 12 population (baseline MMS 4–9).
CS, corticosteroid; ES, endoscopic subscore; fCAL, fecal calprotectin; hsCRP, high-sensitivity C-reactive protein; JAKi, Janus kinase inhibitor; MMS, modified Mayo score; N, number of patients per subgroup; n, number of patients with evaluable data; QD, once daily; SD, standard deviation; UC, ulcerative colitis.
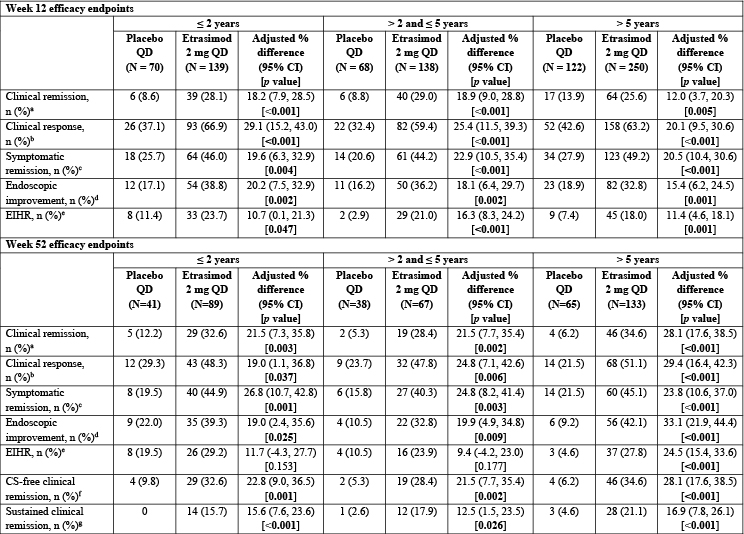
Figure: Table 2. Achievement of efficacy endpoints at Week 12 in the pooled ELEVATE UC 52 and ELEVATE UC 12 patient population, and at Week 52 in ELEVATE UC 52 (baseline MMS 4–9).
p < 0.05 are highlighted in bold. Treatment comparisons (adjusted treatment difference and 95% CI) and 2-sided p values were obtained using the Cochran–Mantel–Haenszel method, adjusting to reported randomization stratification of prior biologic/Janus kinase inhibitor therapy exposure (yes/no), baseline CS use (yes/no), baseline disease activity (MMS of 4–6 or 7–9), and study (for pooled studies only). Missing response was considered as nonresponse.
[a] Clinical remission was defined as SFS = 0 (or = 1 with a ≥ 1-point decrease from baseline), RBS = 0, and ES ≤ 1 (excluding friability).
[b] Clinical response was defined as a ≥ 2-point and ≥ 30% decrease from baseline in MMS, and a ≥ 1-point decrease from baseline in RBS or an absolute RBS ≤ 1.
[c] Symptomatic remission was defined as SFS = 0 (or = 1 with a ≥ 1-point decrease from baseline) and RBS = 0.
[d] Endoscopic improvement was defined as ES ≤ 1.
[e] EIHR was defined as ES ≤ 1 with histologic remission (Geboes Index score < 2.0).
[f] CS-free clinical remission was defined as the proportion of patients with clinical remission at Week 52 who had not been receiving CS for ≥12 weeks immediately prior to Week 52.
[g] Sustained clinical remission was defined as the proportion of patients achieving clinical remission at both Week 12 and Week 52.
CI, confidence intervals; CS, corticosteroid; EIHR, endoscopic improvement-histologic remission; ES, endoscopic subscore; MMS, modified Mayo score; N, number of patients per subgroup; n, number of patients with evaluable data; QD, once daily; RBS, rectal bleeding subscore; SFS, stool frequency subscore; UC, ulcerative colitis.
Disclosures:
Edward Barnes: AbbVie, Inc. – Consultant. Boomerang – Consultant. Eli Lilly – Consultant. Eli Lilly – Grant/Research Support. Pfizer – Consultant. Sanofi – Consultant. Takeda – Consultant. Target RWE – Consultant.
Fernando Magro: AbbVie – Personal Fees. Amgen – Personal Fees. Biogen – Personal Fees. Celgene – Personal Fees. Celltrion – Personal Fees. Dr Falk Pharma – Personal Fees. Ferring – Personal Fees. GEDII – Grant/Research Support. Hospira – Personal Fees. Janssen – Personal Fees. Laboratórios Vitória – Personal Fees. MSD – Personal Fees. National Science Foundation – Grant/Research Support. Pfizer Inc – Personal Fees. Sandoz – Personal Fees. Takeda – Personal Fees. UCB – Personal Fees. Vifor – Personal Fees.
Hiroshi Nakase: AbbVie GK – Grant/Research Support, Speakers Bureau. AbbVie GK – Speakers Bureau. Ayumi Pharmaceutical – Grant/Research Support. EA Pharma – Grant/Research Support. Gilead Sciences – Speakers Bureau. HOYA Pentax Medical – Grant/Research Support. Janssen Pharmaceutical K.K – Speakers Bureau. JIMRO – Endowed Chair, Speakers Bureau. Kyorin Pharmaceutical – Speakers Bureau. Mitsubishi Tanabe Pharma – Grant/Research Support, Speakers Bureau. MIYARISAN Pharmaceutical Co, Ltd – Endowed Chair, Speakers Bureau. Mochida Pharmaceutical – Grant/Research Support, Endowed Chair, Speakers Bureau. Nihon Kayaku Co, Ltd – Grant/Research Support. Otsuka Pharmaceutical – Grant/Research Support. Pfizer Inc – Speakers Bureau. TAIHO Pharmaceutical Co, Ltd – Grant/Research Support. Takeda – Speakers Bureau. VIATRIS Inc – Speakers Bureau.
Raymond K. Cross: AbbVie – Advisory Committee/Board Member, Consultant, Speakers Bureau. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speakers Bureau. CorEvitas – Advisory Committee/Board Member, Consultant. Fresenius Kabi – Advisory Committee/Board Member, Consultant. Genentech – Advisory Committee/Board Member, Consultant. Gilead – Advisory Committee/Board Member, Consultant. IBD Education Group – Advisory Committee/Board Member, Consultant. Janssen – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Magellan Health – Advisory Committee/Board Member, Consultant. Option Care – Advisory Committee/Board Member, Consultant. Pfizer Inc – Advisory Committee/Board Member, Consultant, Speakers Bureau. Pharmacosmos – Advisory Committee/Board Member, Consultant. Samsung Bioepis – Advisory Committee/Board Member, Consultant. Sandoz – Advisory Committee/Board Member, Consultant. Takeda – Grant/Research Support.
Shannon Chang: AbbVie – Consultant. BMS – Ad board. Janssen – Ad board. Lilly – Ad board. Pfizer – Ad board.
Faten Aberra: AbbVie – Grant/Research Support. Janssen – Advisory Committee/Board Member, Consultant, Grant/Research Support. Merck – Grant/Research Support.
Jesse Green: Pfizer Inc – Employee, Stock Options.
Laure Kouyoudjian: Pfizer Inc – Employee, Stock Options.
Abhishek Bhattacharjee: Pfizer Healthcare India Private Ltd. – Employee. Pfizer Inc – Stock Options.
Maria Kudela: Pfizer Inc – Employee, Stock Options.
Martina Goetsch: Pfizer AG – Employee. Pfizer Inc – Stock Options.
Geert D'Haens: AbbVie – Advisor or Review Panel Member, Speakers Bureau. Alimentiv – Advisor or Review Panel Member. AstraZeneca – Advisor or Review Panel Member. Boehringer Ingelheim – Advisor or Review Panel Member, Speakers Bureau. Bristol Myers Squibb – Advisor or Review Panel Member. Celltrion – Advisor or Review Panel Member, Speakers Bureau. Cosmo – Advisor or Review Panel Member. Eli Lilly – Advisor or Review Panel Member, Speakers Bureau. Galapagos – Advisor or Review Panel Member. GSK – Advisor or Review Panel Member. Johnson and Johnson – Advisor or Review Panel Member, Speakers Bureau. Pfizer Inc – Advisor or Review Panel Member, Speakers Bureau. Polpharma – Advisor or Review Panel Member. Prometheus Biosciences – Advisor or Review Panel Member. Takeda – Advisor or Review Panel Member, Speakers Bureau. Tillotts – Advisor or Review Panel Member, Speakers Bureau. Ventryx – Advisor or Review Panel Member.
Edward L. Barnes, MD, MPH1, Fernando Magro, MD, PhD2, Hiroshi Nakase, MD, PhD3, Raymond K. K. Cross, MD, MS, FACG4, Shannon Chang, MD5, Faten Aberra, MD, MSCE6, Jesse Green, MD7, Laure Kouyoudjian, MD, MS7, Abhishek Bhattacharjee, PhD8, Maria Kudela, PhD9, Martina Goetsch, MD10, Geert R. D'Haens, MD, PhD11. P1044 - Efficacy of Etrasimod by Disease Duration: An Analysis of the Phase 3 ELEVATE UC Clinical Program, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Multidisciplinary Inflammatory Bowel Diseases Center, Division of Gastroenterology and Hepatology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA, Chapel Hill, NC; 2CINTESIS@RISE, Faculty of Medicine, University of Porto, 4200-450, Porto, Portugal, Porto, Porto, Portugal; 3Department of Gastroenterology and Hepatology, Sapporo Medical University School of Medicine, Hokkaido, Japan, Sapporo, Hokkaido, Japan; 4Division of Gastroenterology and Hepatology, Melissa L. Posner Institute for Digestive Health & Liver Disease at Mercy Medical Center, Baltimore, MD, USA, Baltimore, MD; 5New York University Langone Health, New York, NY, USA, New York, NY; 6Division of Gastroenterology, Hospital of the University of Pennsylvania, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA, Philadelphia, PA; 7Pfizer Inc, Collegeville, PA, USA, Collegeville, PA; 8Pfizer Healthcare India Private Ltd, Chennai, India, Chennai, Tamil Nadu, India; 9Pfizer Inc, Cambridge, MA, USA, Cambridge, MA; 10Pfizer AG, Zürich, Switzerland, Zürich, Zurich, Switzerland; 11Department of Gastroenterology and Hepatology, Amsterdam University Medical Centers, Amsterdam, The Netherlands, Amsterdam, Noord-Holland, Netherlands
Introduction: Ulcerative colitis (UC) can lead to irreversible damage, highlighting the importance of early intervention. While disease duration impacts response to therapy in Crohn’s disease,1,2 similar evidence in UC is lacking.3 Etrasimod is an oral, once-daily (QD), selective sphingosine 1-phosphate (S1P)1,4,5 receptor modulator for the treatment of moderately to severely active UC. We evaluated the efficacy of etrasimod 2 mg QD vs placebo within disease duration subgroups in the ELEVATE UC clinical program.
Methods: In this post hoc analysis of ELEVATE UC 52 (NCT03945188) and ELEVATE UC 12 (NCT03996369), patients were assigned by disease duration (calculated using the date of diagnosis) into three subgroups: ≤ 2 years, > 2 to ≤ 5 years, > 5 years. In each disease duration subgroup, treatment comparisons and 2-sided p</em> values (reported without adjustment to multiple comparisons) were obtained using the Cochran–Mantel–Haenszel method for clinical remission, clinical response, symptomatic remission, endoscopic improvement, and endoscopic improvement-histologic remission at Week 12 and Week 52, and corticosteroid-free and sustained clinical remission at Week 52.
Results: Baseline characteristics were generally balanced within each subgroup; however, a higher proportion of patients in the ≤ 2 years subgroup were biologic/Janus kinase inhibitor-naïve vs the other two subgroups (Table 1). At Week 12 in all subgroups, greater proportions of patients receiving etrasimod vs placebo met criteria for all endpoints (p < 0.05; Table 2). At Week 52, greater proportions of patients receiving etrasimod vs placebo met criteria for all endpoints in the > 5 years subgroup (p < 0.05), and met criteria for most endpoints in the ≤ 2 years and > 2 to ≤ 5 years subgroups (Table 2).
Discussion: Etrasimod demonstrated significant induction and maintenance efficacy vs placebo in both symptomatic and endoscopic endpoints, irrespective of disease duration. These results highlight the efficacy of etrasimod for patients with shorter or longer duration of UC. Future analyses are needed to compare efficacy across disease duration subgroups and longer-term maintenance of response.
Refs:
1. Ungaro R et al. Aliment Pharmacol Ther 2020; 51: 831–842.
2. Noor NM et al. Lancet Gastroenterol Hepatol 2024; 9: 415–427.
3. Solitano V et al. J Clin Med 2020; 9: 2646.
Pfizer’s generative artificial intelligence tool MAIA was used to assist production of the abstract first draft. Authors reviewed/edited and take responsibility for the content.

Figure: Table 1. Patient demographics and baseline characteristics by disease duration subgroup in the pooled ELEVATE UC 52 and ELEVATE UC 12 population (baseline MMS 4–9).
CS, corticosteroid; ES, endoscopic subscore; fCAL, fecal calprotectin; hsCRP, high-sensitivity C-reactive protein; JAKi, Janus kinase inhibitor; MMS, modified Mayo score; N, number of patients per subgroup; n, number of patients with evaluable data; QD, once daily; SD, standard deviation; UC, ulcerative colitis.

Figure: Table 2. Achievement of efficacy endpoints at Week 12 in the pooled ELEVATE UC 52 and ELEVATE UC 12 patient population, and at Week 52 in ELEVATE UC 52 (baseline MMS 4–9).
p < 0.05 are highlighted in bold. Treatment comparisons (adjusted treatment difference and 95% CI) and 2-sided p values were obtained using the Cochran–Mantel–Haenszel method, adjusting to reported randomization stratification of prior biologic/Janus kinase inhibitor therapy exposure (yes/no), baseline CS use (yes/no), baseline disease activity (MMS of 4–6 or 7–9), and study (for pooled studies only). Missing response was considered as nonresponse.
[a] Clinical remission was defined as SFS = 0 (or = 1 with a ≥ 1-point decrease from baseline), RBS = 0, and ES ≤ 1 (excluding friability).
[b] Clinical response was defined as a ≥ 2-point and ≥ 30% decrease from baseline in MMS, and a ≥ 1-point decrease from baseline in RBS or an absolute RBS ≤ 1.
[c] Symptomatic remission was defined as SFS = 0 (or = 1 with a ≥ 1-point decrease from baseline) and RBS = 0.
[d] Endoscopic improvement was defined as ES ≤ 1.
[e] EIHR was defined as ES ≤ 1 with histologic remission (Geboes Index score < 2.0).
[f] CS-free clinical remission was defined as the proportion of patients with clinical remission at Week 52 who had not been receiving CS for ≥12 weeks immediately prior to Week 52.
[g] Sustained clinical remission was defined as the proportion of patients achieving clinical remission at both Week 12 and Week 52.
CI, confidence intervals; CS, corticosteroid; EIHR, endoscopic improvement-histologic remission; ES, endoscopic subscore; MMS, modified Mayo score; N, number of patients per subgroup; n, number of patients with evaluable data; QD, once daily; RBS, rectal bleeding subscore; SFS, stool frequency subscore; UC, ulcerative colitis.
Disclosures:
Edward Barnes: AbbVie, Inc. – Consultant. Boomerang – Consultant. Eli Lilly – Consultant. Eli Lilly – Grant/Research Support. Pfizer – Consultant. Sanofi – Consultant. Takeda – Consultant. Target RWE – Consultant.
Fernando Magro: AbbVie – Personal Fees. Amgen – Personal Fees. Biogen – Personal Fees. Celgene – Personal Fees. Celltrion – Personal Fees. Dr Falk Pharma – Personal Fees. Ferring – Personal Fees. GEDII – Grant/Research Support. Hospira – Personal Fees. Janssen – Personal Fees. Laboratórios Vitória – Personal Fees. MSD – Personal Fees. National Science Foundation – Grant/Research Support. Pfizer Inc – Personal Fees. Sandoz – Personal Fees. Takeda – Personal Fees. UCB – Personal Fees. Vifor – Personal Fees.
Hiroshi Nakase: AbbVie GK – Grant/Research Support, Speakers Bureau. AbbVie GK – Speakers Bureau. Ayumi Pharmaceutical – Grant/Research Support. EA Pharma – Grant/Research Support. Gilead Sciences – Speakers Bureau. HOYA Pentax Medical – Grant/Research Support. Janssen Pharmaceutical K.K – Speakers Bureau. JIMRO – Endowed Chair, Speakers Bureau. Kyorin Pharmaceutical – Speakers Bureau. Mitsubishi Tanabe Pharma – Grant/Research Support, Speakers Bureau. MIYARISAN Pharmaceutical Co, Ltd – Endowed Chair, Speakers Bureau. Mochida Pharmaceutical – Grant/Research Support, Endowed Chair, Speakers Bureau. Nihon Kayaku Co, Ltd – Grant/Research Support. Otsuka Pharmaceutical – Grant/Research Support. Pfizer Inc – Speakers Bureau. TAIHO Pharmaceutical Co, Ltd – Grant/Research Support. Takeda – Speakers Bureau. VIATRIS Inc – Speakers Bureau.
Raymond K. Cross: AbbVie – Advisory Committee/Board Member, Consultant, Speakers Bureau. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speakers Bureau. CorEvitas – Advisory Committee/Board Member, Consultant. Fresenius Kabi – Advisory Committee/Board Member, Consultant. Genentech – Advisory Committee/Board Member, Consultant. Gilead – Advisory Committee/Board Member, Consultant. IBD Education Group – Advisory Committee/Board Member, Consultant. Janssen – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Magellan Health – Advisory Committee/Board Member, Consultant. Option Care – Advisory Committee/Board Member, Consultant. Pfizer Inc – Advisory Committee/Board Member, Consultant, Speakers Bureau. Pharmacosmos – Advisory Committee/Board Member, Consultant. Samsung Bioepis – Advisory Committee/Board Member, Consultant. Sandoz – Advisory Committee/Board Member, Consultant. Takeda – Grant/Research Support.
Shannon Chang: AbbVie – Consultant. BMS – Ad board. Janssen – Ad board. Lilly – Ad board. Pfizer – Ad board.
Faten Aberra: AbbVie – Grant/Research Support. Janssen – Advisory Committee/Board Member, Consultant, Grant/Research Support. Merck – Grant/Research Support.
Jesse Green: Pfizer Inc – Employee, Stock Options.
Laure Kouyoudjian: Pfizer Inc – Employee, Stock Options.
Abhishek Bhattacharjee: Pfizer Healthcare India Private Ltd. – Employee. Pfizer Inc – Stock Options.
Maria Kudela: Pfizer Inc – Employee, Stock Options.
Martina Goetsch: Pfizer AG – Employee. Pfizer Inc – Stock Options.
Geert D'Haens: AbbVie – Advisor or Review Panel Member, Speakers Bureau. Alimentiv – Advisor or Review Panel Member. AstraZeneca – Advisor or Review Panel Member. Boehringer Ingelheim – Advisor or Review Panel Member, Speakers Bureau. Bristol Myers Squibb – Advisor or Review Panel Member. Celltrion – Advisor or Review Panel Member, Speakers Bureau. Cosmo – Advisor or Review Panel Member. Eli Lilly – Advisor or Review Panel Member, Speakers Bureau. Galapagos – Advisor or Review Panel Member. GSK – Advisor or Review Panel Member. Johnson and Johnson – Advisor or Review Panel Member, Speakers Bureau. Pfizer Inc – Advisor or Review Panel Member, Speakers Bureau. Polpharma – Advisor or Review Panel Member. Prometheus Biosciences – Advisor or Review Panel Member. Takeda – Advisor or Review Panel Member, Speakers Bureau. Tillotts – Advisor or Review Panel Member, Speakers Bureau. Ventryx – Advisor or Review Panel Member.
Edward L. Barnes, MD, MPH1, Fernando Magro, MD, PhD2, Hiroshi Nakase, MD, PhD3, Raymond K. K. Cross, MD, MS, FACG4, Shannon Chang, MD5, Faten Aberra, MD, MSCE6, Jesse Green, MD7, Laure Kouyoudjian, MD, MS7, Abhishek Bhattacharjee, PhD8, Maria Kudela, PhD9, Martina Goetsch, MD10, Geert R. D'Haens, MD, PhD11. P1044 - Efficacy of Etrasimod by Disease Duration: An Analysis of the Phase 3 ELEVATE UC Clinical Program, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
