Sunday Poster Session
Category: IBD
P1037 - Comparative Outcomes of Tofacitinib Initiation in Biologic-Naïve vs Biologic-Experienced Ulcerative Colitis Patients: A Retrospective Cohort Analysis
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Om Patel, MD (he/him/his)
Virtua Health System / Virtua Medical Group
Camden, NJ
Presenting Author(s)
Om Patel, MD1, Abdallah Hussein, MD2, Jashanveer S. Singh Johal, MD1, Yecheskel Schneider, MD, MS3, Islam Rajab, MD4, Elaf Khatib, MS5
1Virtua Health System / Virtua Medical Group, Camden, NJ; 2Virtua Our Lady of Lourdes Hospital, Camden, NJ; 3Virtua Health System, Moorestown, NJ; 4St. Joseph's University Medical Center, Paterson, NJ; 5Thomas Jefferson University, Philadelphia, PA
Introduction: While tofacitinib is approved for moderate-to-severe ulcerative colitis (UC), it remains unclear whether prior exposure to biologic therapies modifies its safety and effectiveness. We compared 6-month risks of complication-related hospitalization and partial colectomy after tofacitinib initiation in biologic-naïve versus biologic-experienced UC patients.
Methods: This retrospective cohort study used the USA TriNetX Collaborative Network to identify adult (≥18 years) UC patients who newly initiated tofacitinib between January 1, 2019, and December 31, 2024. Patients were stratified into biologic-experienced (“non-naïve”) and biologic-naïve cohorts. Propensity-score matching (1:1) balanced age, sex, race, and baseline disease characteristics, yielding 516 patients per group. The primary outcome was hospitalization for UC with complications within six months; the secondary outcome was partial colectomy. Univariable risk ratios (RRs) with 95% confidence intervals (CIs) and P values were calculated in each matched cohort.
Results: After propensity score matching, 516 patients were included in each group. The overall cohort’s mean ± SD age was 45.7 ± 17.3 years, with 49% male and the majority White (76%). Matching achieved good balance across baseline characteristics (all standardized differences < 0.10). The risk of ulcerative colitis related complications was similar between non–biologic-naïve and biologic-naïve patients at 29.07% versus 28.88% (RR 1.007; 95% CI, 0.832–1.219; P = 0.945). Likewise, partial colectomy rates did not differ significantly (4.26% vs 5.23%; RR 0.815; 95% CI, 0.471–1.412; P = 0.464).
Discussion: Among UC patients initiating tofacitinib, prior biologic exposure did not significantly influence 6-month complication-related hospitalization or colectomy rates. These findings support comparable safety and effectiveness of tofacitinib irrespective of biologic history.
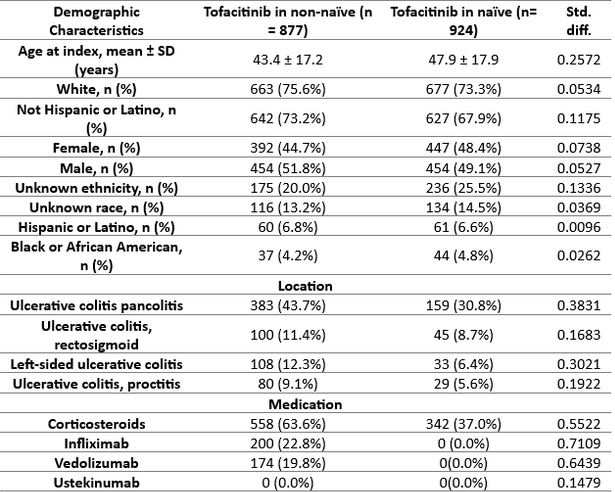
Figure: Demographic Matching Related Outcomes
Disclosures:
Om Patel indicated no relevant financial relationships.
Abdallah Hussein indicated no relevant financial relationships.
Jashanveer Singh Johal indicated no relevant financial relationships.
Yecheskel Schneider indicated no relevant financial relationships.
Islam Rajab indicated no relevant financial relationships.
Elaf Khatib indicated no relevant financial relationships.
Om Patel, MD1, Abdallah Hussein, MD2, Jashanveer S. Singh Johal, MD1, Yecheskel Schneider, MD, MS3, Islam Rajab, MD4, Elaf Khatib, MS5. P1037 - Comparative Outcomes of Tofacitinib Initiation in Biologic-Naïve vs Biologic-Experienced Ulcerative Colitis Patients: A Retrospective Cohort Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Virtua Health System / Virtua Medical Group, Camden, NJ; 2Virtua Our Lady of Lourdes Hospital, Camden, NJ; 3Virtua Health System, Moorestown, NJ; 4St. Joseph's University Medical Center, Paterson, NJ; 5Thomas Jefferson University, Philadelphia, PA
Introduction: While tofacitinib is approved for moderate-to-severe ulcerative colitis (UC), it remains unclear whether prior exposure to biologic therapies modifies its safety and effectiveness. We compared 6-month risks of complication-related hospitalization and partial colectomy after tofacitinib initiation in biologic-naïve versus biologic-experienced UC patients.
Methods: This retrospective cohort study used the USA TriNetX Collaborative Network to identify adult (≥18 years) UC patients who newly initiated tofacitinib between January 1, 2019, and December 31, 2024. Patients were stratified into biologic-experienced (“non-naïve”) and biologic-naïve cohorts. Propensity-score matching (1:1) balanced age, sex, race, and baseline disease characteristics, yielding 516 patients per group. The primary outcome was hospitalization for UC with complications within six months; the secondary outcome was partial colectomy. Univariable risk ratios (RRs) with 95% confidence intervals (CIs) and P values were calculated in each matched cohort.
Results: After propensity score matching, 516 patients were included in each group. The overall cohort’s mean ± SD age was 45.7 ± 17.3 years, with 49% male and the majority White (76%). Matching achieved good balance across baseline characteristics (all standardized differences < 0.10). The risk of ulcerative colitis related complications was similar between non–biologic-naïve and biologic-naïve patients at 29.07% versus 28.88% (RR 1.007; 95% CI, 0.832–1.219; P = 0.945). Likewise, partial colectomy rates did not differ significantly (4.26% vs 5.23%; RR 0.815; 95% CI, 0.471–1.412; P = 0.464).
Discussion: Among UC patients initiating tofacitinib, prior biologic exposure did not significantly influence 6-month complication-related hospitalization or colectomy rates. These findings support comparable safety and effectiveness of tofacitinib irrespective of biologic history.

Figure: Demographic Matching Related Outcomes
Disclosures:
Om Patel indicated no relevant financial relationships.
Abdallah Hussein indicated no relevant financial relationships.
Jashanveer Singh Johal indicated no relevant financial relationships.
Yecheskel Schneider indicated no relevant financial relationships.
Islam Rajab indicated no relevant financial relationships.
Elaf Khatib indicated no relevant financial relationships.
Om Patel, MD1, Abdallah Hussein, MD2, Jashanveer S. Singh Johal, MD1, Yecheskel Schneider, MD, MS3, Islam Rajab, MD4, Elaf Khatib, MS5. P1037 - Comparative Outcomes of Tofacitinib Initiation in Biologic-Naïve vs Biologic-Experienced Ulcerative Colitis Patients: A Retrospective Cohort Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
