Sunday Poster Session
Category: IBD
P1101 - Effectiveness of Combination Biologic And/or Small Molecule Therapies in Inflammatory Bowel Disease: A Single Center Analysis
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Jacob K. Jamison, BA (he/him/his)
Weill Cornell Medicine
New York, NY
Presenting Author(s)
Jacob K. Jamison, BA1, Anjile An, MPH1, Caroline J. Young, MS1, Michael Mintz, MD2, Laura Sahyoun, MD2, Randy S. Longman, MD, PhD2, Vinita Jacob, MD2, Ellen J. Scherl, MD2, Dana J. Lukin, MD, PhD, FACG2
1Weill Cornell Medicine, New York, NY; 2Jill Roberts Center for Inflammatory Bowel Disease, Weill Cornell Medicine, New York, NY
Introduction: Despite an increasing variety of treatment options, many inflammatory bowel disease (IBD) patients do not achieve therapy goals. Plateauing efficacy of single-agent regimens is commonly reported even after therapy optimization. This study evaluates the effectiveness of combination regimens including newer agents in adult IBD patients.
Methods: Adult patients with Crohn’s disease (CD) or ulcerative colitis (UC) treated ≥6 weeks with two concomitant biologic and/or small molecule therapies (including TNF antagonists [TNF-a], anti-integrin agents, IL-(12/)23 inhibitors [IL-12/23i], JAK inhibitors [JAKi], or S1P receptor modulators) were identified via clinical practice and an institutional IBD patient database. Data were obtained on demographics, treatment, and disease activity. For patients who received >1 combination, the first combination was used for demographics. Descriptive statistics are used to report observational outcomes.
Results: 125 regimens among 102 patients (64 CD, 38 UC) were analyzed with 49% female, 9% Black, and 11% Hispanic; mean age was 40 ±17 years. Average disease duration by combination start was 14 ±12 years. 92% received prior steroids and 88% prior TNF-a. 72% failed ≥2 advanced therapies, and 56% of CD patients had prior surgery. Mean baseline Harvey-Bradshaw Index (HBI) and Partial Mayo (PM) were 7 ±7 and 4 ±2, respectively. At baseline, the mean Simple Endoscopic Score for CD was 13 ±10, and 91% of UC patients had a Mayo score of 2 or 3.
Mean combination duration was 12.1 ±12.7 months. Combination IL-12/23i + JAKi (52%) was the most frequent regimen. Among patients with baseline and follow-up HBI/PM data, 74% achieved clinical response and 69% remission. Among patients with endoscopic data, 71% (27/38) achieved improvement and 33% (13/42) remission. Mean time to clinical and endoscopic remission was 4.8 ±4.9 and 10.8 ±11.7 months, respectively. Combinations containing JAKi trended towards the greatest rates of clinical (75%) and endoscopic (40%) remission. Active perianal disease or other extraintestinal manifestations (EIM) improved with 63% and 65% of combinations, respectively. Among patients with elevated CRP (n=40) or fecal calprotectin (n=40), 60% and 63% achieved normalization.
Discussion: In a large specialty IBD center, combination therapy was feasible and effective. No distinct combination achieved significantly superior outcomes. Targeting multiple pathways active in IBD may achieve greater effectiveness than single-agent regimens.
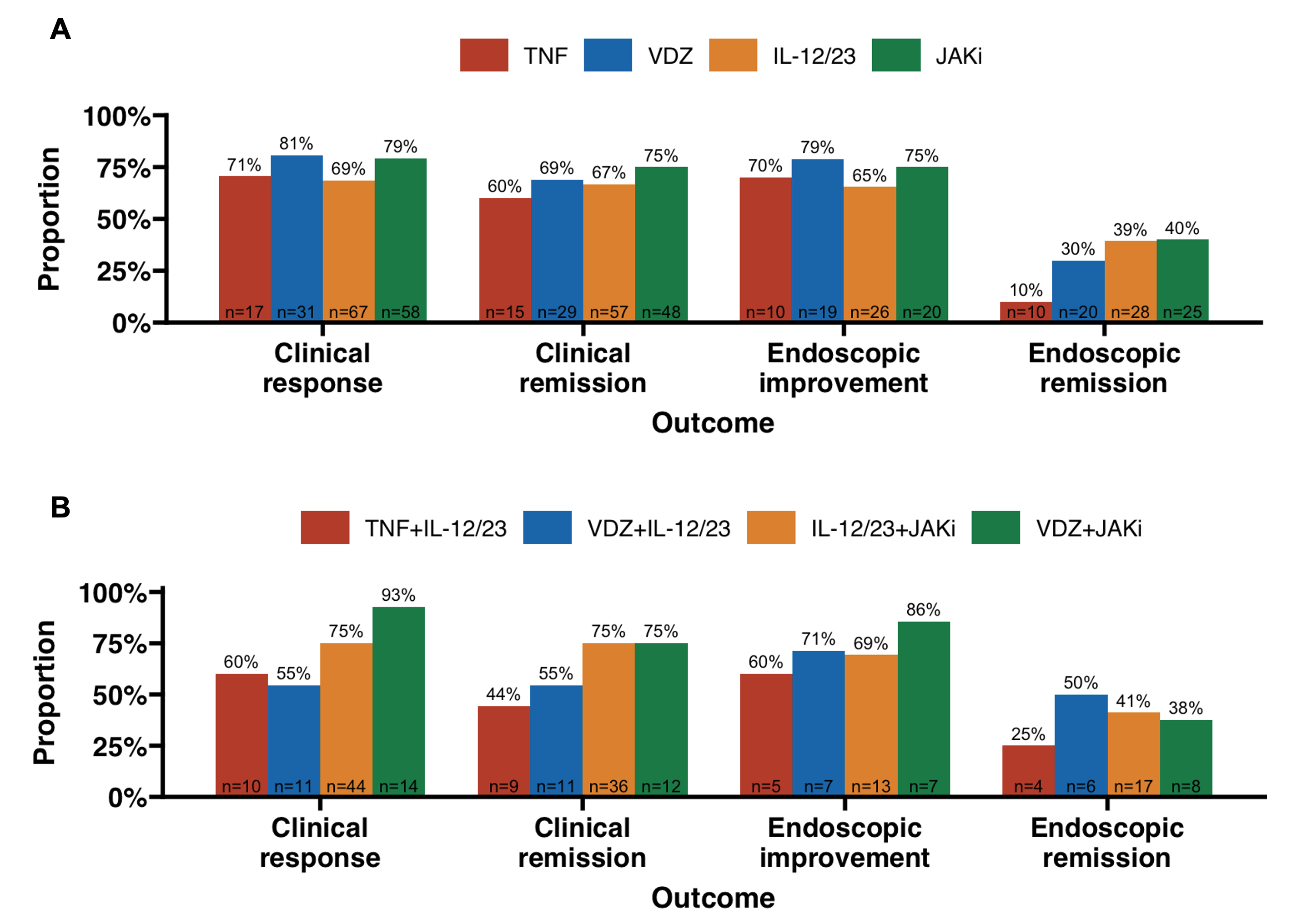
Figure: Figure 1. Clinical and endoscopic response rates by (A) combination containing and (B) specific combination. Abbreviations: TNF, anti-tumor necrosis factor; VDZ, vedolizumab; IL-12/23, interleukin-(12/)23 inhibitor; JAKi, Janus kinase inhibitor.
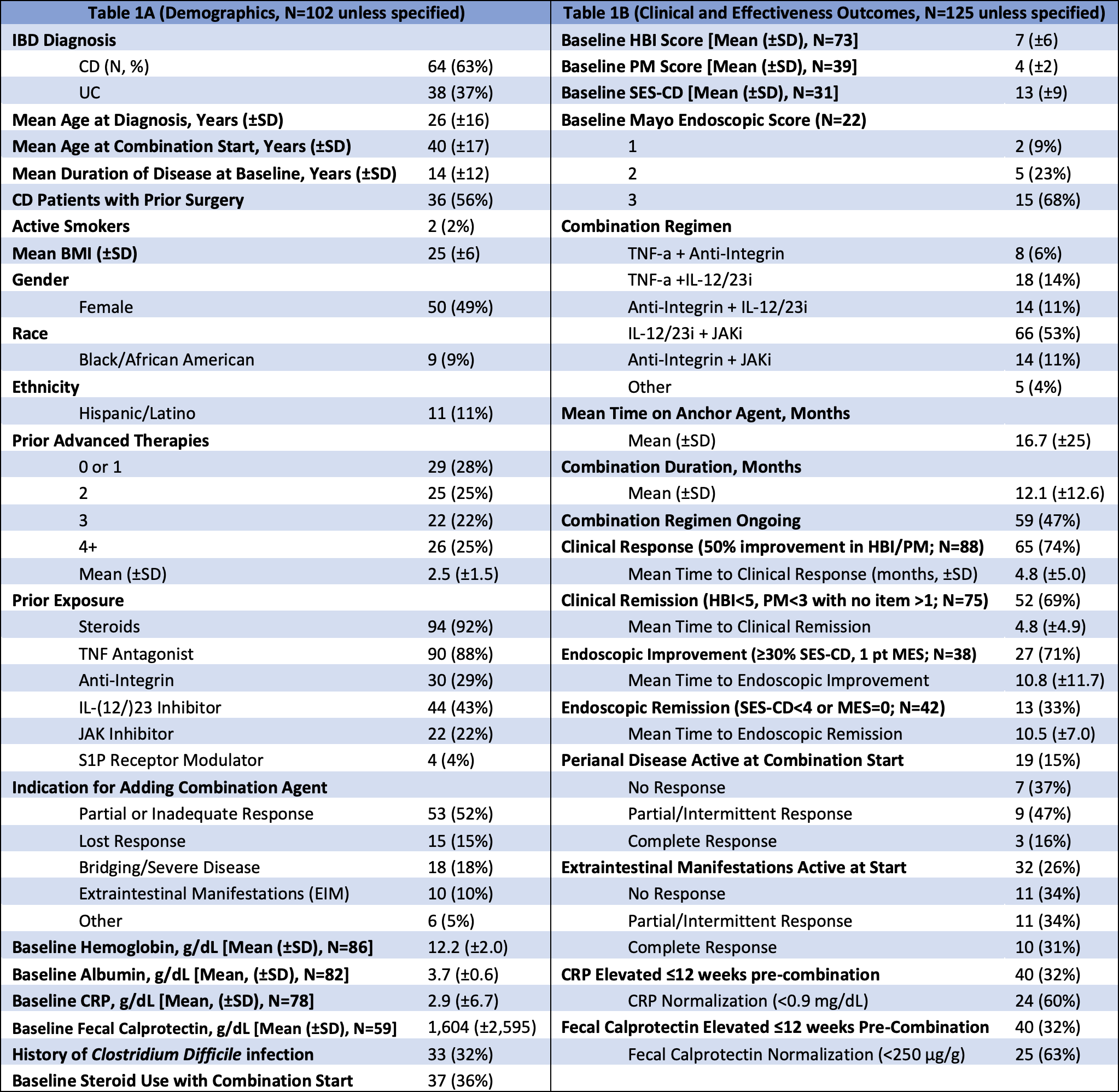
Figure: Table 1. (A) Demographics and (B) clinical and effectiveness outcomes.
Disclosures:
Jacob Jamison indicated no relevant financial relationships.
Anjile An indicated no relevant financial relationships.
Caroline Young indicated no relevant financial relationships.
Michael Mintz indicated no relevant financial relationships.
Laura Sahyoun indicated no relevant financial relationships.
Randy Longman: Ancilia – Advisory Committee/Board Member. Boehringer Ingelheim – Grant/Research Support. CJ Biosciences – Advisory Committee/Board Member. Sanofi – Consultant. Xencor – Consultant.
Vinita Jacob indicated no relevant financial relationships.
Ellen Scherl indicated no relevant financial relationships.
Dana Lukin: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Altrubio – Consultant. Boehringer Ingelheim – Consultant, Grant/Research Support. Bristol Myers Squibb – Consultant. Johnson & Johnson – Consultant, Grant/Research Support, Speakers Bureau. Palatin Technologies – Consultant. Pfizer – Consultant. Prime – Consultant. PSI – Consultant. Takeda – Consultant. Vedanta – Consultant.
Jacob K. Jamison, BA1, Anjile An, MPH1, Caroline J. Young, MS1, Michael Mintz, MD2, Laura Sahyoun, MD2, Randy S. Longman, MD, PhD2, Vinita Jacob, MD2, Ellen J. Scherl, MD2, Dana J. Lukin, MD, PhD, FACG2. P1101 - Effectiveness of Combination Biologic And/or Small Molecule Therapies in Inflammatory Bowel Disease: A Single Center Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Weill Cornell Medicine, New York, NY; 2Jill Roberts Center for Inflammatory Bowel Disease, Weill Cornell Medicine, New York, NY
Introduction: Despite an increasing variety of treatment options, many inflammatory bowel disease (IBD) patients do not achieve therapy goals. Plateauing efficacy of single-agent regimens is commonly reported even after therapy optimization. This study evaluates the effectiveness of combination regimens including newer agents in adult IBD patients.
Methods: Adult patients with Crohn’s disease (CD) or ulcerative colitis (UC) treated ≥6 weeks with two concomitant biologic and/or small molecule therapies (including TNF antagonists [TNF-a], anti-integrin agents, IL-(12/)23 inhibitors [IL-12/23i], JAK inhibitors [JAKi], or S1P receptor modulators) were identified via clinical practice and an institutional IBD patient database. Data were obtained on demographics, treatment, and disease activity. For patients who received >1 combination, the first combination was used for demographics. Descriptive statistics are used to report observational outcomes.
Results: 125 regimens among 102 patients (64 CD, 38 UC) were analyzed with 49% female, 9% Black, and 11% Hispanic; mean age was 40 ±17 years. Average disease duration by combination start was 14 ±12 years. 92% received prior steroids and 88% prior TNF-a. 72% failed ≥2 advanced therapies, and 56% of CD patients had prior surgery. Mean baseline Harvey-Bradshaw Index (HBI) and Partial Mayo (PM) were 7 ±7 and 4 ±2, respectively. At baseline, the mean Simple Endoscopic Score for CD was 13 ±10, and 91% of UC patients had a Mayo score of 2 or 3.
Mean combination duration was 12.1 ±12.7 months. Combination IL-12/23i + JAKi (52%) was the most frequent regimen. Among patients with baseline and follow-up HBI/PM data, 74% achieved clinical response and 69% remission. Among patients with endoscopic data, 71% (27/38) achieved improvement and 33% (13/42) remission. Mean time to clinical and endoscopic remission was 4.8 ±4.9 and 10.8 ±11.7 months, respectively. Combinations containing JAKi trended towards the greatest rates of clinical (75%) and endoscopic (40%) remission. Active perianal disease or other extraintestinal manifestations (EIM) improved with 63% and 65% of combinations, respectively. Among patients with elevated CRP (n=40) or fecal calprotectin (n=40), 60% and 63% achieved normalization.
Discussion: In a large specialty IBD center, combination therapy was feasible and effective. No distinct combination achieved significantly superior outcomes. Targeting multiple pathways active in IBD may achieve greater effectiveness than single-agent regimens.

Figure: Figure 1. Clinical and endoscopic response rates by (A) combination containing and (B) specific combination. Abbreviations: TNF, anti-tumor necrosis factor; VDZ, vedolizumab; IL-12/23, interleukin-(12/)23 inhibitor; JAKi, Janus kinase inhibitor.

Figure: Table 1. (A) Demographics and (B) clinical and effectiveness outcomes.
Disclosures:
Jacob Jamison indicated no relevant financial relationships.
Anjile An indicated no relevant financial relationships.
Caroline Young indicated no relevant financial relationships.
Michael Mintz indicated no relevant financial relationships.
Laura Sahyoun indicated no relevant financial relationships.
Randy Longman: Ancilia – Advisory Committee/Board Member. Boehringer Ingelheim – Grant/Research Support. CJ Biosciences – Advisory Committee/Board Member. Sanofi – Consultant. Xencor – Consultant.
Vinita Jacob indicated no relevant financial relationships.
Ellen Scherl indicated no relevant financial relationships.
Dana Lukin: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Altrubio – Consultant. Boehringer Ingelheim – Consultant, Grant/Research Support. Bristol Myers Squibb – Consultant. Johnson & Johnson – Consultant, Grant/Research Support, Speakers Bureau. Palatin Technologies – Consultant. Pfizer – Consultant. Prime – Consultant. PSI – Consultant. Takeda – Consultant. Vedanta – Consultant.
Jacob K. Jamison, BA1, Anjile An, MPH1, Caroline J. Young, MS1, Michael Mintz, MD2, Laura Sahyoun, MD2, Randy S. Longman, MD, PhD2, Vinita Jacob, MD2, Ellen J. Scherl, MD2, Dana J. Lukin, MD, PhD, FACG2. P1101 - Effectiveness of Combination Biologic And/or Small Molecule Therapies in Inflammatory Bowel Disease: A Single Center Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

