Sunday Poster Session
Category: IBD
P1092 - Shared Genetic Risk and Disease Overlap in the Gut-Lung Axis: A Systematic Review and Meta-Analysis of Inflammatory Bowel Disease and Pulmonary Comorbidity
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Samuel Huang, MD
Mount Sinai South Nassau - One Health WayOceanside, NY 11572UNITED STATES - Oceanside, NY
Oceanside, NY
Presenting Author(s)
Samuel Huang, MD1, Jessica Su, MD2, Alexander Huang, MD3
1Mount Sinai South Nassau - One Health WayOceanside, NY 11572UNITED STATES - Oceanside, NY, Oceanside, NY; 2Kaohsiung Medical University, Sanmin District, Kaohsiung, Taiwan; 3Mayo Clinic, Rochester, MN
Introduction: The gut–lung axis represents a key interface in immune-mediated disease, with inflammatory bowel disease (IBD), which encompasses Crohn's disease (CD) and ulcerative colitis (UC), being frequently comorbid with asthma and chronic obstructive pulmonary disease (COPD). While observational data suggest increased comorbidity, it remains unclear whether shared genetic susceptibility explains this overlap. We performed a systematic review and quantitative synthesis to determine the contribution of overlapping genetic loci to disease overlap between gastrointestinal and pulmonary inflammatory conditions.
Methods: We performed a systematic review of GWAS and clinical cohort studies to identify genes associated with both IBD and pulmonary disease. Using publicly available odds ratios (ORs) and allele frequencies, we estimated gene-specific adjusted disease prevalence for asthma, UC, and CD. Expected overlap prevalence due to shared genetic risk was calculated using a probabilistic model. These were contrasted with observed comorbidity rates derived from U.S. Nationwide Inpatient Sample (NIS) datasets. Meta-analyses were conducted where there were multiple studies available per gene, and forest plots were generated to display effect sizes and heterogeneity.
Results: Five major genes (ORMDL3, IL23R, NOD2, DENND1B, and SMAD3) had dual associations. ORMDL3 was highly associated with asthma (OR 1.44, 95% CI: 1.35–1.54) and more weakly with UC (OR 1.12, 95% CI: 0.98–1.27). NOD2 was strongly associated with CD (OR 2.20, 95% CI: 1.84–2.62) and modestly with asthma (OR 1.10, 95% CI: 1.00–1.22). Despite these, the genetically expected disease overlap (e.g., ORMDL3-mediated asthma–UC overlap: 0.0018%) was much lower than the observed overlap (~8%) in hospitalized patients. Forest plots demonstrated wide heterogeneity, with I² ranging from 35% to 70% for various genes.
Discussion: While several genetic loci are associated with both pulmonary and gastrointestinal inflammatory diseases, the anticipated overlap via genetic risk alone is lower than the observed real-world comorbidity burden. This implicates additional factors—among them shared environmental stimuli, loss of epithelial barrier function, mucosal immunity, and microbiome disruption—in potentially propagating the gut–lung axis. These findings underscore the need for exploration of gene–environment interaction and for controlling for comorbidity in clinical risk prediction and therapeutic targeting.
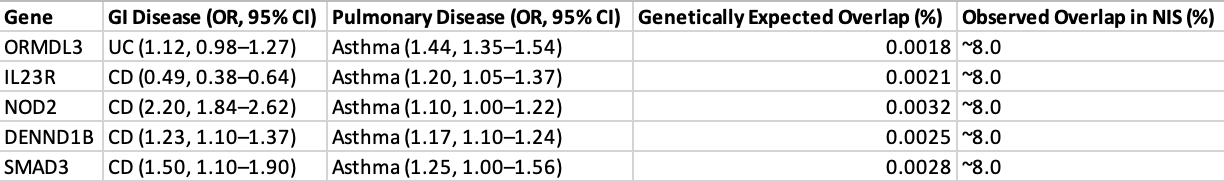
Figure: This table presents odds ratios (ORs) and 95% confidence intervals (CIs) for genetic variants implicated in Crohn’s disease, asthma, and chronic obstructive pulmonary disease (COPD). For each locus, disease-specific associations are listed alongside the original study references from which effect estimates were derived. Odds ratios were calculated within the context of the individual disease phenotype and should not be interpreted as cross-disease estimates.
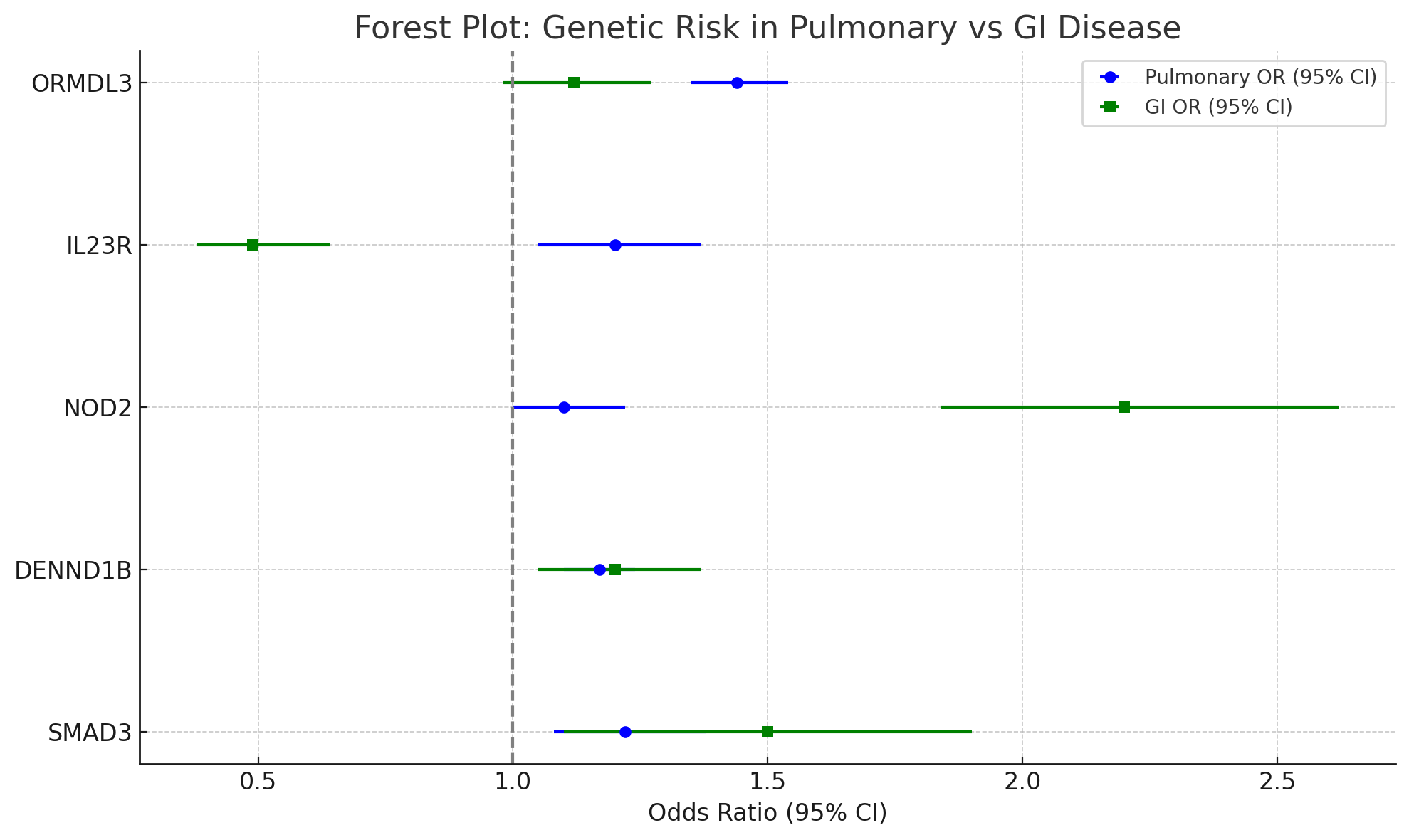
Figure: Table 1. Comparison of Odds Ratios (ORs) of Shared Genetic Loci in GI and Pulmonary Diseases. The following table summarizes the published genome-wide association studies (GWAS) concerning five genetic loci associations with gastrointestinal (GI) and pulmonary diseases. Odds ratios (ORs) and 95% confidence intervals (CIs) for each gene are reported for inflammatory bowel diseases, such as Crohn's disease (CD) or ulcerative colitis (UC) and for respiratory diseases, primarily asthma and COPD. Probabilistically anticipated overlap in disease co-occurrence was determined according to a model integrating ORs and allele frequencies. These estimates are contrasted with the documented comorbidity prevalence (~8%) reported in the U.S. Nationwide Inpatient Sample (NIS), emphasizing the contrast between gene-based prediction and true disease burden. ORs indicate increased (OR >1) or decreased (OR <1) risk, and predicted overlap percentages quantify the magnitude of dual diagnoses based on shared genetic risk only.
Disclosures:
Samuel Huang indicated no relevant financial relationships.
Jessica Su indicated no relevant financial relationships.
Alexander Huang indicated no relevant financial relationships.
Samuel Huang, MD1, Jessica Su, MD2, Alexander Huang, MD3. P1092 - Shared Genetic Risk and Disease Overlap in the Gut-Lung Axis: A Systematic Review and Meta-Analysis of Inflammatory Bowel Disease and Pulmonary Comorbidity, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Mount Sinai South Nassau - One Health WayOceanside, NY 11572UNITED STATES - Oceanside, NY, Oceanside, NY; 2Kaohsiung Medical University, Sanmin District, Kaohsiung, Taiwan; 3Mayo Clinic, Rochester, MN
Introduction: The gut–lung axis represents a key interface in immune-mediated disease, with inflammatory bowel disease (IBD), which encompasses Crohn's disease (CD) and ulcerative colitis (UC), being frequently comorbid with asthma and chronic obstructive pulmonary disease (COPD). While observational data suggest increased comorbidity, it remains unclear whether shared genetic susceptibility explains this overlap. We performed a systematic review and quantitative synthesis to determine the contribution of overlapping genetic loci to disease overlap between gastrointestinal and pulmonary inflammatory conditions.
Methods: We performed a systematic review of GWAS and clinical cohort studies to identify genes associated with both IBD and pulmonary disease. Using publicly available odds ratios (ORs) and allele frequencies, we estimated gene-specific adjusted disease prevalence for asthma, UC, and CD. Expected overlap prevalence due to shared genetic risk was calculated using a probabilistic model. These were contrasted with observed comorbidity rates derived from U.S. Nationwide Inpatient Sample (NIS) datasets. Meta-analyses were conducted where there were multiple studies available per gene, and forest plots were generated to display effect sizes and heterogeneity.
Results: Five major genes (ORMDL3, IL23R, NOD2, DENND1B, and SMAD3) had dual associations. ORMDL3 was highly associated with asthma (OR 1.44, 95% CI: 1.35–1.54) and more weakly with UC (OR 1.12, 95% CI: 0.98–1.27). NOD2 was strongly associated with CD (OR 2.20, 95% CI: 1.84–2.62) and modestly with asthma (OR 1.10, 95% CI: 1.00–1.22). Despite these, the genetically expected disease overlap (e.g., ORMDL3-mediated asthma–UC overlap: 0.0018%) was much lower than the observed overlap (~8%) in hospitalized patients. Forest plots demonstrated wide heterogeneity, with I² ranging from 35% to 70% for various genes.
Discussion: While several genetic loci are associated with both pulmonary and gastrointestinal inflammatory diseases, the anticipated overlap via genetic risk alone is lower than the observed real-world comorbidity burden. This implicates additional factors—among them shared environmental stimuli, loss of epithelial barrier function, mucosal immunity, and microbiome disruption—in potentially propagating the gut–lung axis. These findings underscore the need for exploration of gene–environment interaction and for controlling for comorbidity in clinical risk prediction and therapeutic targeting.

Figure: This table presents odds ratios (ORs) and 95% confidence intervals (CIs) for genetic variants implicated in Crohn’s disease, asthma, and chronic obstructive pulmonary disease (COPD). For each locus, disease-specific associations are listed alongside the original study references from which effect estimates were derived. Odds ratios were calculated within the context of the individual disease phenotype and should not be interpreted as cross-disease estimates.

Figure: Table 1. Comparison of Odds Ratios (ORs) of Shared Genetic Loci in GI and Pulmonary Diseases. The following table summarizes the published genome-wide association studies (GWAS) concerning five genetic loci associations with gastrointestinal (GI) and pulmonary diseases. Odds ratios (ORs) and 95% confidence intervals (CIs) for each gene are reported for inflammatory bowel diseases, such as Crohn's disease (CD) or ulcerative colitis (UC) and for respiratory diseases, primarily asthma and COPD. Probabilistically anticipated overlap in disease co-occurrence was determined according to a model integrating ORs and allele frequencies. These estimates are contrasted with the documented comorbidity prevalence (~8%) reported in the U.S. Nationwide Inpatient Sample (NIS), emphasizing the contrast between gene-based prediction and true disease burden. ORs indicate increased (OR >1) or decreased (OR <1) risk, and predicted overlap percentages quantify the magnitude of dual diagnoses based on shared genetic risk only.
Disclosures:
Samuel Huang indicated no relevant financial relationships.
Jessica Su indicated no relevant financial relationships.
Alexander Huang indicated no relevant financial relationships.
Samuel Huang, MD1, Jessica Su, MD2, Alexander Huang, MD3. P1092 - Shared Genetic Risk and Disease Overlap in the Gut-Lung Axis: A Systematic Review and Meta-Analysis of Inflammatory Bowel Disease and Pulmonary Comorbidity, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
