Sunday Poster Session
Category: IBD
P1089 - Reigniting the Response When Maintenance Fails: Real-World Outcomes of Upadacitinib Dose Escalation
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Abigail L. Ellington, MD (she/her/hers)
Atrium Health Wake Forest Baptist
Winston-Salem, NC
Presenting Author(s)
Abigail L. Ellington, MD1, Danielle Rambuss, MD1, Sarah Barbina, MD1, Maithili V. Chitnavis, MD, FACG2
1Atrium Health Wake Forest Baptist, Winston-Salem, NC; 2Atrium Health, Charlotte, NC
Introduction: Upadacitinib is a small molecule selective janus kinase (JAK) 1 inhibitor used in treating IBD. FDA approval limits the initial induction dosing of 45 mg for 8 weeks for ulcerative colitis (UC) and 12 weeks for Crohn’s disease (CD), followed by maintenance dosing of 15 or 30 mg. Some patients with UC or CD lose response to recommended maintenance doses. Currently, no data exists on dose re-escalation in these cases. This descriptive cohort study examines reasons for continuation of or re-escalation to induction dosing and clinical response.
Methods: We conducted a retrospective single-center cohort study of 57 patients who received off-label escalated dosing of upadacitinib between February 2023 and March 2025. Inclusion required at least one re-induction cycle of the higher regimen. Patient demographics, gastrointestinal symptoms, and pre- and post-treatment outcomes were collected from the electronic health record.
Results: Among 57 patients, the average age was 37.6 years, 58% were male, and 66.7% had CD. Most (53) received dose escalation after insufficient response on maintenance dosing. Patients were on 2.6 (mean) other biologics prior to upadacitinib. Escalation was prompted by GI symptoms in 31 patients, lab findings in 8, and active endoscopic disease in 27. GI symptoms improved as shown in Figure 1. Mean FCP dropped from 678 to 392 (38.8% decrease) in 21 patients in whom measurements were available. Nine patients had a simple endoscopic score for Crohn’s disease (SES-CD) before and after the dose change; average SES-CD dropped from 10.2 to 7.2. Thirteen patients were started on combination therapy with another biologic agent to achieve disease remission despite dose escalation. Seven patients stopped upadacitinib due to adverse reaction (n=2), ineffectiveness (n=4), or cancer treatment (n=1).
Discussion: This study demonstrates the improvement in symptoms, FCP, and SES-CD with dose escalation of upadacitinib. Limitations of this study include variations in clinical practice regarding the timing of follow-up labs and endoscopy, as well as documentation of symptoms. Additionally, some patients previously on escalated dosing may have been missed if they were off the drug at the time of data analysis. Prospective studies with defined protocols for off-label dose escalation during maintenance therapy should be pursued, as our single-center study suggests a role for escalated maintenance dosing of upadacitinib in improving subjective and objective markers of disease in IBD patients.
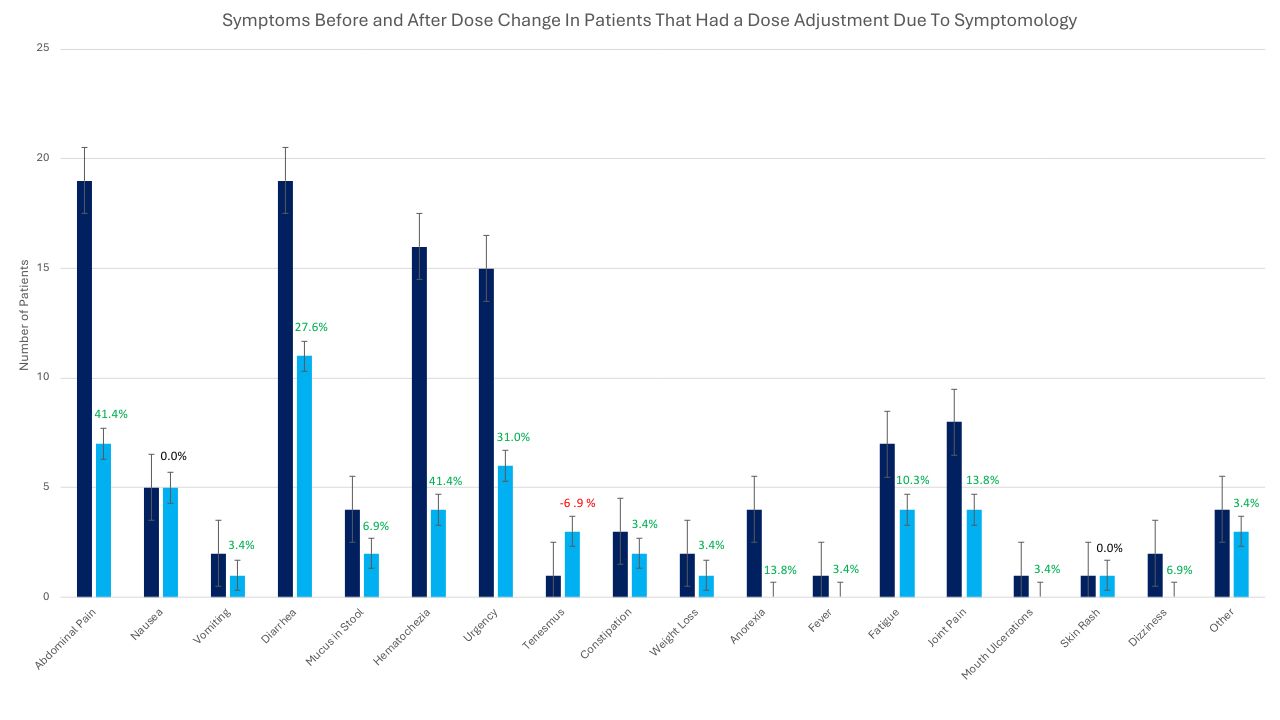
Figure: Figure [1]. Symptom prevalence before and after upadacitinib dose escalation. This bar graph illustrates the number of patients reporting each symptom before and after dose escalation of upadacitinib. Symptoms assessed include abdominal pain, nausea, vomiting, diarrhea, mucus in stool, hematochezia, urgency, tenesmus, constipation, weight loss, anorexia, fever, fatigue, joint pain, mouth ulcerations, skin rash, dizziness, and other. Percent change in symptom frequency is also shown; a negative percent change indicates an increase in symptom prevalence following dose escalation (observed only for tenesmus). All other symptoms showed improvement after dose adjustment. Analysis included 29 of the 31 patients who were escalated due to uncontrolled symptoms; two patients were excluded due to lack of a follow-up office visit post–dose escalation.
Disclosures:
Abigail Ellington indicated no relevant financial relationships.
Danielle Rambuss indicated no relevant financial relationships.
Sarah Barbina indicated no relevant financial relationships.
Maithili Chitnavis: AbbVie – Advisory Committee/Board Member, Speakers Bureau. Eli Lilly – Speakers Bureau.
Abigail L. Ellington, MD1, Danielle Rambuss, MD1, Sarah Barbina, MD1, Maithili V. Chitnavis, MD, FACG2. P1089 - Reigniting the Response When Maintenance Fails: Real-World Outcomes of Upadacitinib Dose Escalation, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Atrium Health Wake Forest Baptist, Winston-Salem, NC; 2Atrium Health, Charlotte, NC
Introduction: Upadacitinib is a small molecule selective janus kinase (JAK) 1 inhibitor used in treating IBD. FDA approval limits the initial induction dosing of 45 mg for 8 weeks for ulcerative colitis (UC) and 12 weeks for Crohn’s disease (CD), followed by maintenance dosing of 15 or 30 mg. Some patients with UC or CD lose response to recommended maintenance doses. Currently, no data exists on dose re-escalation in these cases. This descriptive cohort study examines reasons for continuation of or re-escalation to induction dosing and clinical response.
Methods: We conducted a retrospective single-center cohort study of 57 patients who received off-label escalated dosing of upadacitinib between February 2023 and March 2025. Inclusion required at least one re-induction cycle of the higher regimen. Patient demographics, gastrointestinal symptoms, and pre- and post-treatment outcomes were collected from the electronic health record.
Results: Among 57 patients, the average age was 37.6 years, 58% were male, and 66.7% had CD. Most (53) received dose escalation after insufficient response on maintenance dosing. Patients were on 2.6 (mean) other biologics prior to upadacitinib. Escalation was prompted by GI symptoms in 31 patients, lab findings in 8, and active endoscopic disease in 27. GI symptoms improved as shown in Figure 1. Mean FCP dropped from 678 to 392 (38.8% decrease) in 21 patients in whom measurements were available. Nine patients had a simple endoscopic score for Crohn’s disease (SES-CD) before and after the dose change; average SES-CD dropped from 10.2 to 7.2. Thirteen patients were started on combination therapy with another biologic agent to achieve disease remission despite dose escalation. Seven patients stopped upadacitinib due to adverse reaction (n=2), ineffectiveness (n=4), or cancer treatment (n=1).
Discussion: This study demonstrates the improvement in symptoms, FCP, and SES-CD with dose escalation of upadacitinib. Limitations of this study include variations in clinical practice regarding the timing of follow-up labs and endoscopy, as well as documentation of symptoms. Additionally, some patients previously on escalated dosing may have been missed if they were off the drug at the time of data analysis. Prospective studies with defined protocols for off-label dose escalation during maintenance therapy should be pursued, as our single-center study suggests a role for escalated maintenance dosing of upadacitinib in improving subjective and objective markers of disease in IBD patients.

Figure: Figure [1]. Symptom prevalence before and after upadacitinib dose escalation. This bar graph illustrates the number of patients reporting each symptom before and after dose escalation of upadacitinib. Symptoms assessed include abdominal pain, nausea, vomiting, diarrhea, mucus in stool, hematochezia, urgency, tenesmus, constipation, weight loss, anorexia, fever, fatigue, joint pain, mouth ulcerations, skin rash, dizziness, and other. Percent change in symptom frequency is also shown; a negative percent change indicates an increase in symptom prevalence following dose escalation (observed only for tenesmus). All other symptoms showed improvement after dose adjustment. Analysis included 29 of the 31 patients who were escalated due to uncontrolled symptoms; two patients were excluded due to lack of a follow-up office visit post–dose escalation.
Disclosures:
Abigail Ellington indicated no relevant financial relationships.
Danielle Rambuss indicated no relevant financial relationships.
Sarah Barbina indicated no relevant financial relationships.
Maithili Chitnavis: AbbVie – Advisory Committee/Board Member, Speakers Bureau. Eli Lilly – Speakers Bureau.
Abigail L. Ellington, MD1, Danielle Rambuss, MD1, Sarah Barbina, MD1, Maithili V. Chitnavis, MD, FACG2. P1089 - Reigniting the Response When Maintenance Fails: Real-World Outcomes of Upadacitinib Dose Escalation, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
