Sunday Poster Session
Category: IBD
P1178 - Early Results of a New Intestinal Ultrasound Program as a Real-Time Disease Monitoring Tool in Patients With Ulcerative Colitis: A Retrospective Case Series
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
- NE
Nabil El Hage Chehade, MD
Scripps Clinic
San Diego, CA
Presenting Author(s)
Nabil El Hage Chehade, MD1, Tara Alleyasin, MD2, Mazer Ally, MD3, Rebecca Matro, MD4, Gauree Konijeti, MD, MPH, FACG5
1Scripps Clinic, San Diego, CA; 2Scripps Green Hospital, San Diego, CA; 3Scripps Clinic Medical Group, San Diego, CA; 4Scripps Health, San Diego, CA; 5Scripps Clinic Medical Group, La Jolla, CA
Introduction: Intestinal ultrasound (IUS) is a non-invasive and inexpensive point-of-care disease monitoring tool increasingly utilized in the management of inflammatory bowel disease (IBD). Data regarding its efficacy in US-based IBD centers newly implementing an IUS program are limited. The aim of our study was to assess the accuracy of IUS in the management of ulcerative colitis (UC) patients within our institution’s new IUS program.
Methods: We conducted a retrospective case series of adult patients >18 years old with UC who underwent IUS between July and November 2024 at Scripps Clinic. Indications for IUS were to assess disease activity or response to therapy. Data on clinical disease activity scores, endoscopic/radiographic findings, and fecal calprotectin (FC) within 6 months of IUS were collected. The primary endpoint of the study was to assess the correlation between IUS findings and recent clinical, endoscopic, and biochemical disease state. The secondary endpoint was to evaluate the extent to which IUS aided in the management of UC patients.
Results: A total of 40 adult patients (mean age=43.5 years, male gender=16) with UC (pancolonic=24 (60%), left colon=16 (40%)) completed IUS during the designated study period. None had prior abdominal surgeries. 45% (18/40) of patients failed at least 1 prior biologic therapy and 53% (21/40) of patients were on biologic therapy when IUS was performed. IUS examination revealed radiographic remission in 50% (20/40) of patients, while 43% (17/40) and 8% (3/40) of patients had mild-moderate left-sided colitis and pancolitis, respectively. IUS findings were concordant with clinical symptoms in 95% (38/40) of patients. IUS findings were also concordant with recent luminal disease extent/severity by colonoscopy/flexible sigmoidoscopy and FC in 100% (18/18) and 81.5% (22/27) of cases, respectively. The use of IUS aided in therapy escalation in 11 patients (27.5%), de-escalation in 5 patients (12.5%), and continuation of the same therapy in 24 patients (60%).
Discussion: Our study demonstrates that early integration of IUS in an IBD program shows accurate correlation with symptoms and objective disease measures for UC and aids in clinical decision making. IUS provided valuable real-time data, which led to therapy modification in almost 40% of included patients. Data collection of IUS performed on UC patients at our institution in the past 12 months is currently underway, final report to follow.
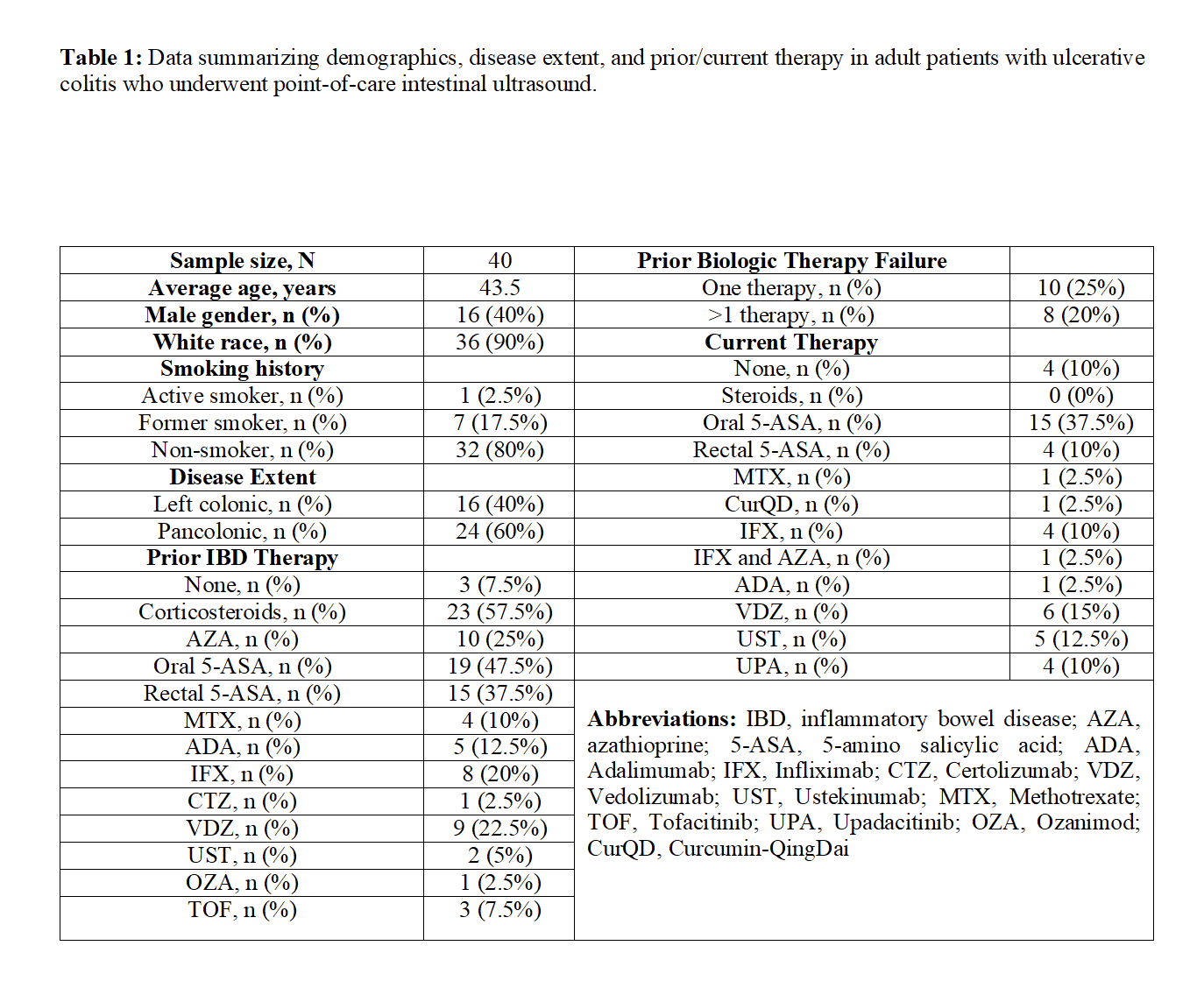
Figure: Table 1: Data summarizing demographics, disease extent, and prior/current therapy in adult patients with ulcerative colitis who underwent point-of-care intestinal ultrasound.
Disclosures:
Nabil El Hage Chehade indicated no relevant financial relationships.
Tara Alleyasin indicated no relevant financial relationships.
Mazer Ally: Abbvie – Speakers Bureau. Lilly – Advisory Committee/Board Member.
Rebecca Matro indicated no relevant financial relationships.
Gauree Konijeti: Abbvie – Advisory Committee/Board Member, Consultant. Johnson and Johnson – Consultant. Lilly – Consultant, Speakers Bureau. Pfizer – Advisory Committee/Board Member. Takeda – Speakers Bureau. WellTheory – Consultant, Stock Options.
Nabil El Hage Chehade, MD1, Tara Alleyasin, MD2, Mazer Ally, MD3, Rebecca Matro, MD4, Gauree Konijeti, MD, MPH, FACG5. P1178 - Early Results of a New Intestinal Ultrasound Program as a Real-Time Disease Monitoring Tool in Patients With Ulcerative Colitis: A Retrospective Case Series, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Scripps Clinic, San Diego, CA; 2Scripps Green Hospital, San Diego, CA; 3Scripps Clinic Medical Group, San Diego, CA; 4Scripps Health, San Diego, CA; 5Scripps Clinic Medical Group, La Jolla, CA
Introduction: Intestinal ultrasound (IUS) is a non-invasive and inexpensive point-of-care disease monitoring tool increasingly utilized in the management of inflammatory bowel disease (IBD). Data regarding its efficacy in US-based IBD centers newly implementing an IUS program are limited. The aim of our study was to assess the accuracy of IUS in the management of ulcerative colitis (UC) patients within our institution’s new IUS program.
Methods: We conducted a retrospective case series of adult patients >18 years old with UC who underwent IUS between July and November 2024 at Scripps Clinic. Indications for IUS were to assess disease activity or response to therapy. Data on clinical disease activity scores, endoscopic/radiographic findings, and fecal calprotectin (FC) within 6 months of IUS were collected. The primary endpoint of the study was to assess the correlation between IUS findings and recent clinical, endoscopic, and biochemical disease state. The secondary endpoint was to evaluate the extent to which IUS aided in the management of UC patients.
Results: A total of 40 adult patients (mean age=43.5 years, male gender=16) with UC (pancolonic=24 (60%), left colon=16 (40%)) completed IUS during the designated study period. None had prior abdominal surgeries. 45% (18/40) of patients failed at least 1 prior biologic therapy and 53% (21/40) of patients were on biologic therapy when IUS was performed. IUS examination revealed radiographic remission in 50% (20/40) of patients, while 43% (17/40) and 8% (3/40) of patients had mild-moderate left-sided colitis and pancolitis, respectively. IUS findings were concordant with clinical symptoms in 95% (38/40) of patients. IUS findings were also concordant with recent luminal disease extent/severity by colonoscopy/flexible sigmoidoscopy and FC in 100% (18/18) and 81.5% (22/27) of cases, respectively. The use of IUS aided in therapy escalation in 11 patients (27.5%), de-escalation in 5 patients (12.5%), and continuation of the same therapy in 24 patients (60%).
Discussion: Our study demonstrates that early integration of IUS in an IBD program shows accurate correlation with symptoms and objective disease measures for UC and aids in clinical decision making. IUS provided valuable real-time data, which led to therapy modification in almost 40% of included patients. Data collection of IUS performed on UC patients at our institution in the past 12 months is currently underway, final report to follow.

Figure: Table 1: Data summarizing demographics, disease extent, and prior/current therapy in adult patients with ulcerative colitis who underwent point-of-care intestinal ultrasound.
Disclosures:
Nabil El Hage Chehade indicated no relevant financial relationships.
Tara Alleyasin indicated no relevant financial relationships.
Mazer Ally: Abbvie – Speakers Bureau. Lilly – Advisory Committee/Board Member.
Rebecca Matro indicated no relevant financial relationships.
Gauree Konijeti: Abbvie – Advisory Committee/Board Member, Consultant. Johnson and Johnson – Consultant. Lilly – Consultant, Speakers Bureau. Pfizer – Advisory Committee/Board Member. Takeda – Speakers Bureau. WellTheory – Consultant, Stock Options.
Nabil El Hage Chehade, MD1, Tara Alleyasin, MD2, Mazer Ally, MD3, Rebecca Matro, MD4, Gauree Konijeti, MD, MPH, FACG5. P1178 - Early Results of a New Intestinal Ultrasound Program as a Real-Time Disease Monitoring Tool in Patients With Ulcerative Colitis: A Retrospective Case Series, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
