Sunday Poster Session
Category: IBD
P1172 - Assessing Trial Eligibility and Real-World Response to Vedolizumab in Patients With Ulcerative Colitis
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Tasnim Ahmed, MD
Beth Israel Deaconess Medical Center
Boston, MA
Presenting Author(s)
Tasnim Ahmed, MD, Aditya Mithal, MD, PhD, Stephen Le Breton, MD, Jini Huh, PharmD, Konstantinos Papamichail, MD, PhD, Adam Cheifetz, MD, FACG
Beth Israel Deaconess Medical Center, Boston, MA
Introduction: Biologics have changed treatment options for patients with inflammatory bowel disease (IBD). These medications were approved via large randomized controlled trials (RCTs) with strict criteria for patient enrollment, which is an ongoing challenge for the field. Ongoing assessment is necessary to assess whether RCT eligibility criteria is adequately reflective of real-world clinical practice as new IBD medications are approved. Here, we investigate the trial-eligibility and clinical outcomes of patients with ulcerative colitis (UC) started on vedolizumab (VDZ) therapy.
Methods: This was a single center retrospective cohort study including patients with UC who received a new prescription for VDZ at a large academic IBD referral center between January, 2020 and June, 2023. The primary outcome was to assess the trial eligibility of patients using the inclusion and exclusion criteria from the GEMINI-1 RCT, while the secondary outcome was to investigate the time to treatment failure (survival analysis), defined as drug discontinuation due to primary non-response, loss of response, or a serious adverse event, as well as the need for an IBD-related surgery or the addition of another biologic. Patients were followed from start of VDZ until treatment failure or the end of the follow up period (May, 2024).
Results: 164 patients who received a new VDZ prescription during the study period were reviewed for inclusion. The final study population consisted of 77 patients with UC (Figure 1). Only 12 out of 77 patients (15.6%) were trial eligible. The most common reasons for exclusion from trial eligibility were topical therapy either with 5-ASA or corticosteroids (39%) followed by medical comorbidities (19.5%). Patients were followed for a median of 18.9 (interquartile range: 12.3-32.9) months. Overall, 21/65 (32.3%) ineligible compared to 6/12 (50%) eligible patients had a treatment failure (Figure 2).
Discussion: This study showed that most patients who were started on VDZ would not have qualified for inclusion in the GEMINI-1 RCT, largely due to topical therapy and comorbidities. In fact, exposure to topical therapy within 14 days remains an exclusion criterion even in the latest RCTs, including INSPIRE, U-ACHIEVE and U-ACCOMPLISH. Changes to these trial eligibility criteria may be warranted to improve external validity and enable recruitment of a diverse patient population that is more representative of real-world conditions.
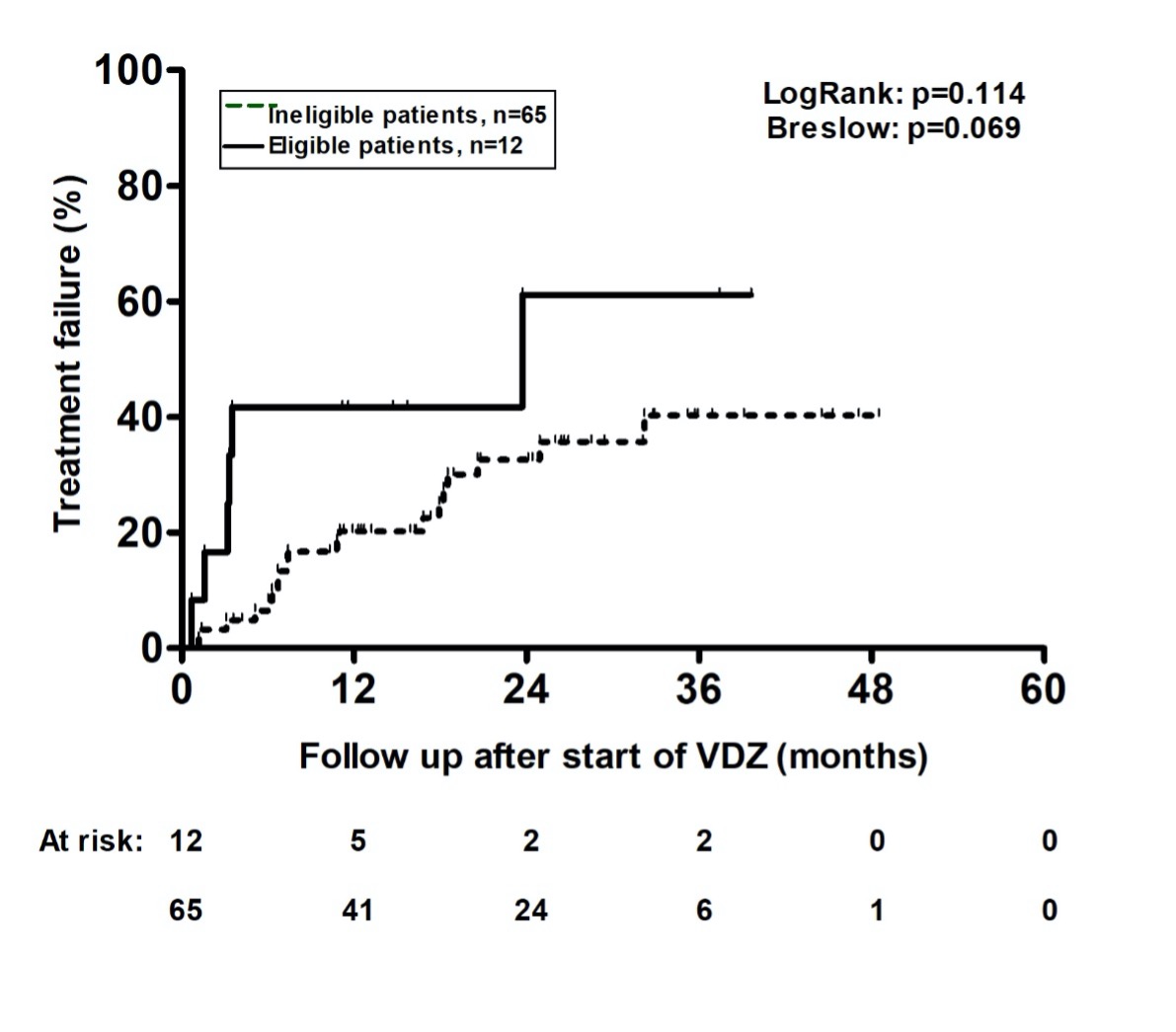
Figure: Figure 2: Kaplan–Meier cumulative probability curves of treatment failure in patients with UC for eligible (solid line) or ineligible (dotted line) for the GEMINI-1 randomized controlled trial.

Figure: Figure 1. Baseline clinical and demographic characteristics of the study population.
Disclosures:
Tasnim Ahmed indicated no relevant financial relationships.
Aditya Mithal indicated no relevant financial relationships.
Stephen Le Breton indicated no relevant financial relationships.
Jini Huh indicated no relevant financial relationships.
Konstantinos Papamichail: Celltrion Inc – Advisory Committee/Board Member. Prometheus Laboratories Inc – Consultant, Speakers Bureau.
Adam Cheifetz: Abbvie – Consultant, Speakers Bureau. Adiso – Consultant. Aegirbio – Advisory Committee/Board Member. Artizan – Advisory Committee/Board Member. BMS – Consultant, Speakers Bureau. Celltrion Inc – Advisory Committee/Board Member, Consultant. Clario – Consultant. Eli Lilly – Consultant. Food is Good – Consultant. Fresenius Kabi – Consultant. Fzata – Consultant. Johnson and Johnson – Consultant, Speakers Bureau. Pfizer – Consultant. ProciseDx – Advisory Committee/Board Member. Prometheus – Advisory Committee/Board Member. Samsung – Consultant. Spherix – Consultant.
Tasnim Ahmed, MD, Aditya Mithal, MD, PhD, Stephen Le Breton, MD, Jini Huh, PharmD, Konstantinos Papamichail, MD, PhD, Adam Cheifetz, MD, FACG. P1172 - Assessing Trial Eligibility and Real-World Response to Vedolizumab in Patients With Ulcerative Colitis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Beth Israel Deaconess Medical Center, Boston, MA
Introduction: Biologics have changed treatment options for patients with inflammatory bowel disease (IBD). These medications were approved via large randomized controlled trials (RCTs) with strict criteria for patient enrollment, which is an ongoing challenge for the field. Ongoing assessment is necessary to assess whether RCT eligibility criteria is adequately reflective of real-world clinical practice as new IBD medications are approved. Here, we investigate the trial-eligibility and clinical outcomes of patients with ulcerative colitis (UC) started on vedolizumab (VDZ) therapy.
Methods: This was a single center retrospective cohort study including patients with UC who received a new prescription for VDZ at a large academic IBD referral center between January, 2020 and June, 2023. The primary outcome was to assess the trial eligibility of patients using the inclusion and exclusion criteria from the GEMINI-1 RCT, while the secondary outcome was to investigate the time to treatment failure (survival analysis), defined as drug discontinuation due to primary non-response, loss of response, or a serious adverse event, as well as the need for an IBD-related surgery or the addition of another biologic. Patients were followed from start of VDZ until treatment failure or the end of the follow up period (May, 2024).
Results: 164 patients who received a new VDZ prescription during the study period were reviewed for inclusion. The final study population consisted of 77 patients with UC (Figure 1). Only 12 out of 77 patients (15.6%) were trial eligible. The most common reasons for exclusion from trial eligibility were topical therapy either with 5-ASA or corticosteroids (39%) followed by medical comorbidities (19.5%). Patients were followed for a median of 18.9 (interquartile range: 12.3-32.9) months. Overall, 21/65 (32.3%) ineligible compared to 6/12 (50%) eligible patients had a treatment failure (Figure 2).
Discussion: This study showed that most patients who were started on VDZ would not have qualified for inclusion in the GEMINI-1 RCT, largely due to topical therapy and comorbidities. In fact, exposure to topical therapy within 14 days remains an exclusion criterion even in the latest RCTs, including INSPIRE, U-ACHIEVE and U-ACCOMPLISH. Changes to these trial eligibility criteria may be warranted to improve external validity and enable recruitment of a diverse patient population that is more representative of real-world conditions.

Figure: Figure 2: Kaplan–Meier cumulative probability curves of treatment failure in patients with UC for eligible (solid line) or ineligible (dotted line) for the GEMINI-1 randomized controlled trial.

Figure: Figure 1. Baseline clinical and demographic characteristics of the study population.
Disclosures:
Tasnim Ahmed indicated no relevant financial relationships.
Aditya Mithal indicated no relevant financial relationships.
Stephen Le Breton indicated no relevant financial relationships.
Jini Huh indicated no relevant financial relationships.
Konstantinos Papamichail: Celltrion Inc – Advisory Committee/Board Member. Prometheus Laboratories Inc – Consultant, Speakers Bureau.
Adam Cheifetz: Abbvie – Consultant, Speakers Bureau. Adiso – Consultant. Aegirbio – Advisory Committee/Board Member. Artizan – Advisory Committee/Board Member. BMS – Consultant, Speakers Bureau. Celltrion Inc – Advisory Committee/Board Member, Consultant. Clario – Consultant. Eli Lilly – Consultant. Food is Good – Consultant. Fresenius Kabi – Consultant. Fzata – Consultant. Johnson and Johnson – Consultant, Speakers Bureau. Pfizer – Consultant. ProciseDx – Advisory Committee/Board Member. Prometheus – Advisory Committee/Board Member. Samsung – Consultant. Spherix – Consultant.
Tasnim Ahmed, MD, Aditya Mithal, MD, PhD, Stephen Le Breton, MD, Jini Huh, PharmD, Konstantinos Papamichail, MD, PhD, Adam Cheifetz, MD, FACG. P1172 - Assessing Trial Eligibility and Real-World Response to Vedolizumab in Patients With Ulcerative Colitis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
