Sunday Poster Session
Category: IBD
P1170 - Impact of Age on Anti-Drug Antibody Formation in Patients With Inflammatory Bowel Disease Treated With Tumor Necrosis Factor Inhibitors
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
- DC
David B. Cao, MD
University of Chicago Medicine
Chicago, IL
Presenting Author(s)
David B. Cao, MD1, Aaron Goffinet, MD2, Noa Krugliak Cleveland, MD3, Russell D. Cohen, MD1, Sushila Dalal, MD2, Benjamin McDonald, MD, PhD3, Joel Pekow, MD3, David T. Rubin, MD4, Tenzin Choden, MD2
1University of Chicago Medicine, Chicago, IL; 2University of Chicago Medicine, Inflammatory Bowel Disease Center, Chicago, IL; 3University of Chicago, Chicago, IL; 4University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL, USA, Chicago, IL
Introduction: It is not known how the risk of immunogenicity to monoclonal antibodies that treat inflammatory bowel disease (IBD) changes with advancing age. Our study investigated whether older adults exhibit different rates of anti-drug antibody (ADA) formation to tumor necrosis factor (TNF) inhibitors.
Methods: We conducted a single-center retrospective study in patients ages 18-89 seen between 2017-2024 with a diagnosis of ulcerative colitis (UC) or Crohn’s disease (CD) receiving anti-TNF medication. Multiple logistic regression was used to evaluate independent associations between clinically relevant ADA (crADA), defined as positive ADA with concurrent free serum drug level below threshold levels (adalimumab < 7.5 ug/mL, infliximab < 5 ug/mL, certolizumab < 20 ug/mL, golimumab < 2 ug/mL), and age, anti-TNF dosing, history of autoimmune disease (see Table 1), current or prior smoking history, IBD type (UC or CD), and concomitant immunomodulator (methotrexate or thiopurine) use.
Results: We identified 925 patients who underwent 1,509 ADA tests. Of these, 969 tests had associated drug dosing information and were included in the final analysis. 91 ADA tests were performed on 68 older adults (ages 60-89y), and 878 tests were performed on 587 younger adults (ages 18-59y). Older adults undergoing ADA testing were more likely to be current or prior smokers (49% vs 26%, p< 0.01) compared to younger adults (Table 1).
24% of tests in older adults showed crADA compared with 20% in younger adults (p = 0.33). In multivariate analysis, being an older adult was not associated with crADA formation (OR 1.45, 95% CI 0.85-2.45) (Figure 1), nor was age when analyzed as a continuous variable (OR 1.01, 95% CI 0.99-1.02).
Escalated anti-TNF dosing (Table 1) was associated with decreased rate of crADA (OR 0.43, 95% CI 0.31-0.60). Other factors such as autoimmune disease (OR 1.44, 95% CI 0.91-2.29), smoking history (OR 0.81, 95% CI 0.56-1.18), and diagnosis of CD (OR 1.39, 95% CI 0.92-2.09) were not associated with crADA formation. When escalated drug dosing was added to the model, concomitant immunomodulator use was also not independently associated with crADA formation (OR 0.8, 95% CI 0.57-1.12).
Discussion: Older adults with IBD demonstrated similar rates of crADA formation to anti-TNF compared to younger adults, indicating chronological age alone does not influence ADA development. Decisions regarding ADA prevention and management should be guided by clinical context rather than chronological age.
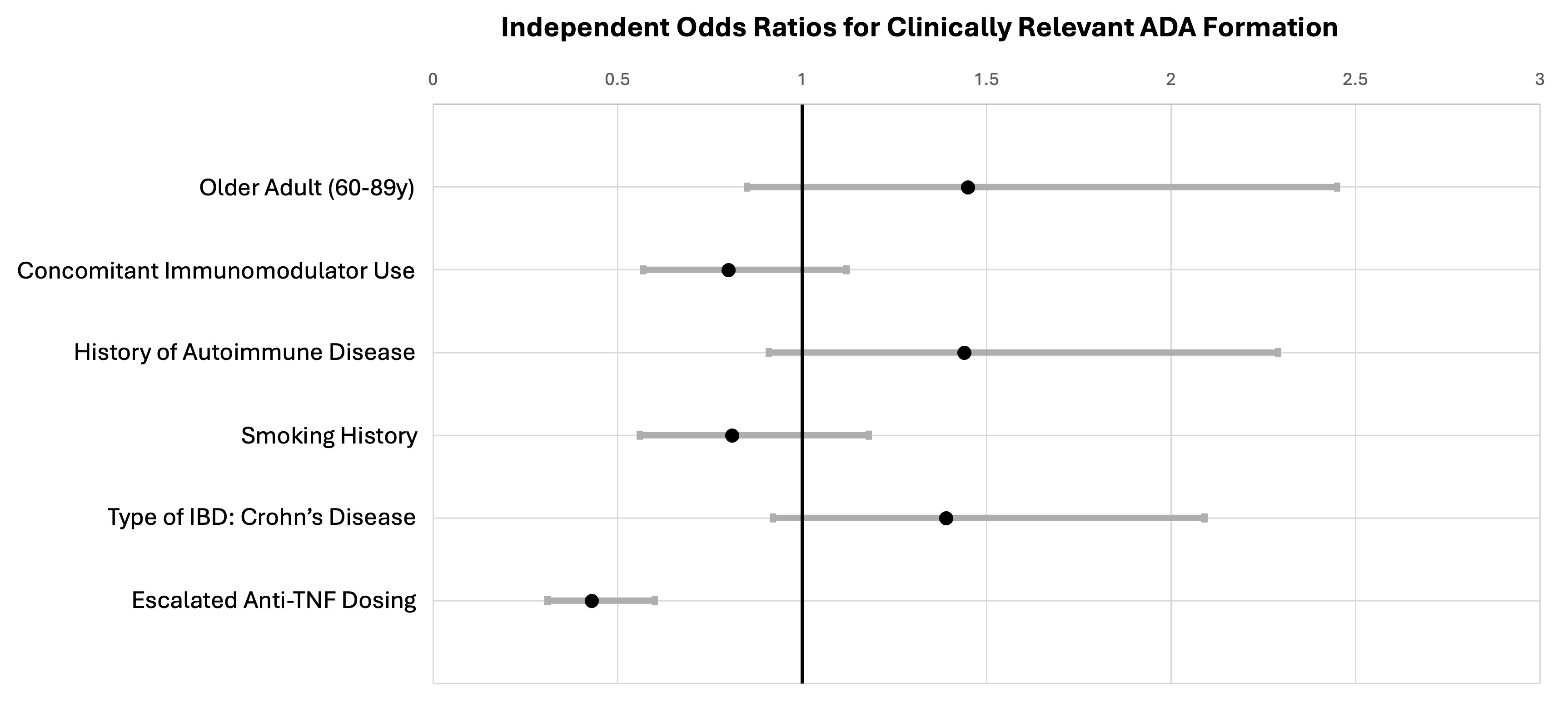
Figure: Table 1: Characteristics of patients undergoing ADA testing.
* Clinically relevant ADA defined as positive ADA with concurrent free serum drug level below therapeutic level. Therapeutic drug levels used: adalimumab < 7.5 ug/mL, infliximab < 5 ug/mL, certolizumab < 20 ug/mL, golimumab < 2 ug/mL
**Autoimmune disease includes ankylosing spondylitis, peripheral arthritis, psoriatic arthritis, psoriasis, erythema nodosum, pyoderma gangrenosum, primary sclerosing cholangitis, autoimmune hepatitis, Hashimoto's thyroiditis, Graves' disease, type 1 diabetes mellitus, uveitis, episcleritis, autoimmune hemolytic anemia, systemic lupus erythematosus, multiple sclerosis, celiac disease
***Standard drug dosing defined as maintenance regimens: adalimumab 40 mg every 2 weeks, infliximab 5 mg/kg every 8 weeks, certolizumab 400 mg every 4 weeks, golimumab 100 mg every 4 weeks. Escalated dosing defined as any maintenance regimen above standard in dose or frequency.
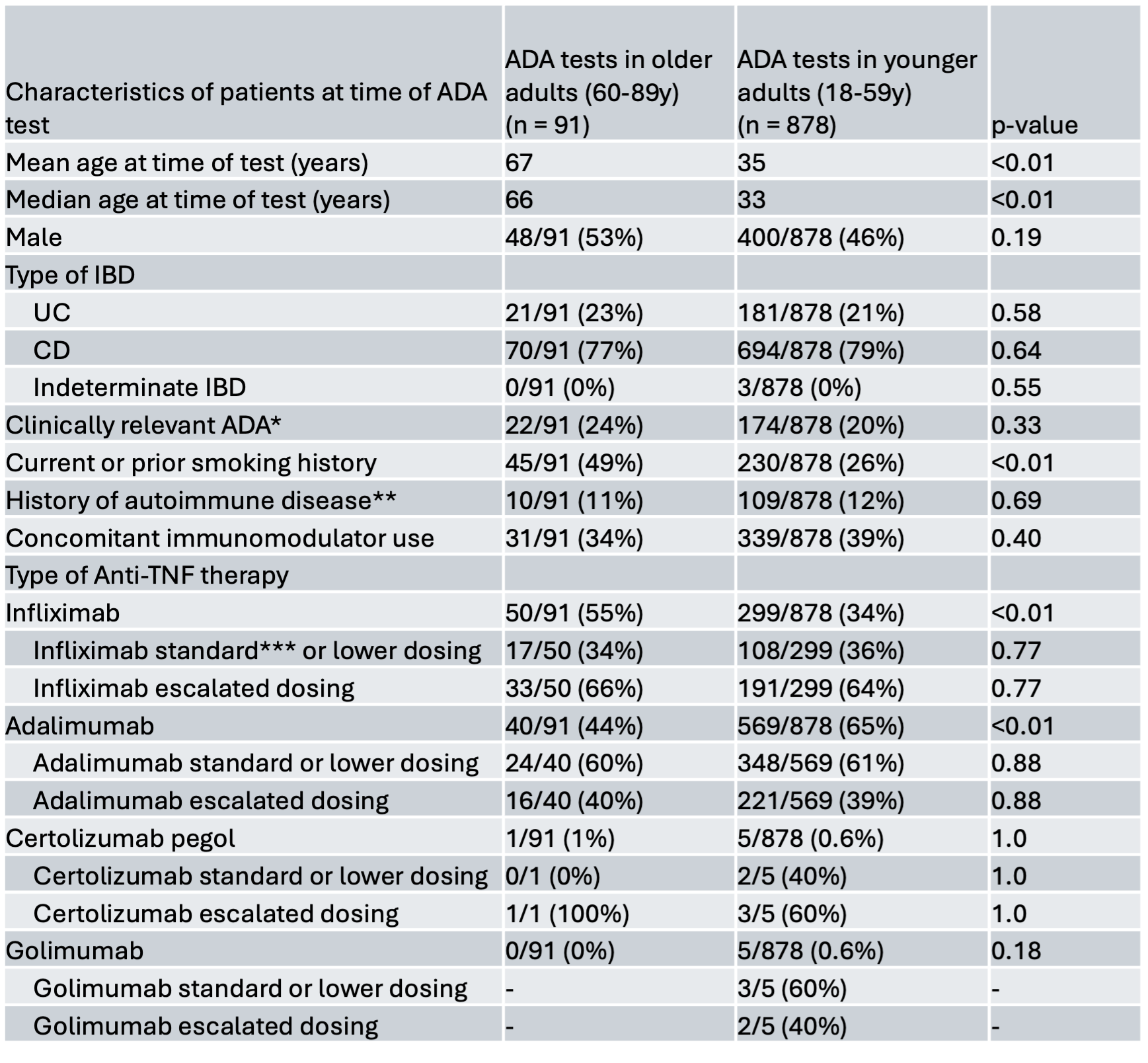
Figure: Figure 1: Forest plot of independent odds ratios and 95% confidence intervals for associations between selected variables and clinically relevant ADA formation
Disclosures:
David Cao indicated no relevant financial relationships.
Aaron Goffinet indicated no relevant financial relationships.
Noa Krugliak Cleveland: Johnson & Johnson – Consultant. NueroLogica – Consultant.
Russell Cohen: Abbvie – Consultant, Speakers Bureau. Bausch Health – Consultant. BMS – Consultant. Eli lilly – Consultant. Genentech – Consultant. Gilead Sciences – Consultant. Johnson & Johnson – Consultant. Pfizer – Consultant. Takeda – Consultant.
Sushila Dalal: Abbvie – Speakers Bureau.
Benjamin McDonald: Iterative Health – Consultant.
Joel Pekow: Abbvie – Stock-publicly held company(excluding mutual/index funds). CVS Health – Consultant. Eli Lilly – Stock-publicly held company(excluding mutual/index funds). Johnson and Johnson – Stock-publicly held company(excluding mutual/index funds). Pfizer – Stock-publicly held company(excluding mutual/index funds).
David Rubin: AbbVie – Advisory Committee/Board Member, Consultant, Speaker fees. Abivax SA – Consultant. Altrubio – Advisory Committee/Board Member, Consultant, Speaker feees, Stock Options. Avalo – Advisory Committee/Board Member, Consultant, Speaker fees. Bausch Health – Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speaker fees. Buhlmann Diagnostics – Advisory Committee/Board Member, Consultant, Speaker fees. Celltrion – Consultant. ClostraBio – Consultant. Connect BioPharma – Consultant. Cornerstones Health, Inc – Board of Directors membership. Douglas Pharmaceuticals – Consultant. Eli Lilly & Co. – Consultant. Foresee, Genentech (Roche) Inc. – Consultant. Image Analysis Group – Consultant. InDex Pharmaceutical – Consultant. Intouch Group – Advisory Committee/Board Member, Consultant, Speaker fees. Iterative Health – Advisory Committee/Board Member, Consultant, Speaker fees. Iterative Health – Stock Options. Janssen Pharmaceuticals – Consultant. Lilly – Advisory Committee/Board Member, Consultant, Speaker fees. Odyssey Therapeutics – Consultant. Pfizer – Advisory Committee/Board Member, Consultant, Speaker fees. Sanofi – Consultant. Takeda – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speaker fees. Throne – Consultant. Vedanta – Consultant.
Tenzin Choden indicated no relevant financial relationships.
David B. Cao, MD1, Aaron Goffinet, MD2, Noa Krugliak Cleveland, MD3, Russell D. Cohen, MD1, Sushila Dalal, MD2, Benjamin McDonald, MD, PhD3, Joel Pekow, MD3, David T. Rubin, MD4, Tenzin Choden, MD2. P1170 - Impact of Age on Anti-Drug Antibody Formation in Patients With Inflammatory Bowel Disease Treated With Tumor Necrosis Factor Inhibitors, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Chicago Medicine, Chicago, IL; 2University of Chicago Medicine, Inflammatory Bowel Disease Center, Chicago, IL; 3University of Chicago, Chicago, IL; 4University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL, USA, Chicago, IL
Introduction: It is not known how the risk of immunogenicity to monoclonal antibodies that treat inflammatory bowel disease (IBD) changes with advancing age. Our study investigated whether older adults exhibit different rates of anti-drug antibody (ADA) formation to tumor necrosis factor (TNF) inhibitors.
Methods: We conducted a single-center retrospective study in patients ages 18-89 seen between 2017-2024 with a diagnosis of ulcerative colitis (UC) or Crohn’s disease (CD) receiving anti-TNF medication. Multiple logistic regression was used to evaluate independent associations between clinically relevant ADA (crADA), defined as positive ADA with concurrent free serum drug level below threshold levels (adalimumab < 7.5 ug/mL, infliximab < 5 ug/mL, certolizumab < 20 ug/mL, golimumab < 2 ug/mL), and age, anti-TNF dosing, history of autoimmune disease (see Table 1), current or prior smoking history, IBD type (UC or CD), and concomitant immunomodulator (methotrexate or thiopurine) use.
Results: We identified 925 patients who underwent 1,509 ADA tests. Of these, 969 tests had associated drug dosing information and were included in the final analysis. 91 ADA tests were performed on 68 older adults (ages 60-89y), and 878 tests were performed on 587 younger adults (ages 18-59y). Older adults undergoing ADA testing were more likely to be current or prior smokers (49% vs 26%, p< 0.01) compared to younger adults (Table 1).
24% of tests in older adults showed crADA compared with 20% in younger adults (p = 0.33). In multivariate analysis, being an older adult was not associated with crADA formation (OR 1.45, 95% CI 0.85-2.45) (Figure 1), nor was age when analyzed as a continuous variable (OR 1.01, 95% CI 0.99-1.02).
Escalated anti-TNF dosing (Table 1) was associated with decreased rate of crADA (OR 0.43, 95% CI 0.31-0.60). Other factors such as autoimmune disease (OR 1.44, 95% CI 0.91-2.29), smoking history (OR 0.81, 95% CI 0.56-1.18), and diagnosis of CD (OR 1.39, 95% CI 0.92-2.09) were not associated with crADA formation. When escalated drug dosing was added to the model, concomitant immunomodulator use was also not independently associated with crADA formation (OR 0.8, 95% CI 0.57-1.12).
Discussion: Older adults with IBD demonstrated similar rates of crADA formation to anti-TNF compared to younger adults, indicating chronological age alone does not influence ADA development. Decisions regarding ADA prevention and management should be guided by clinical context rather than chronological age.

Figure: Table 1: Characteristics of patients undergoing ADA testing.
* Clinically relevant ADA defined as positive ADA with concurrent free serum drug level below therapeutic level. Therapeutic drug levels used: adalimumab < 7.5 ug/mL, infliximab < 5 ug/mL, certolizumab < 20 ug/mL, golimumab < 2 ug/mL
**Autoimmune disease includes ankylosing spondylitis, peripheral arthritis, psoriatic arthritis, psoriasis, erythema nodosum, pyoderma gangrenosum, primary sclerosing cholangitis, autoimmune hepatitis, Hashimoto's thyroiditis, Graves' disease, type 1 diabetes mellitus, uveitis, episcleritis, autoimmune hemolytic anemia, systemic lupus erythematosus, multiple sclerosis, celiac disease
***Standard drug dosing defined as maintenance regimens: adalimumab 40 mg every 2 weeks, infliximab 5 mg/kg every 8 weeks, certolizumab 400 mg every 4 weeks, golimumab 100 mg every 4 weeks. Escalated dosing defined as any maintenance regimen above standard in dose or frequency.

Figure: Figure 1: Forest plot of independent odds ratios and 95% confidence intervals for associations between selected variables and clinically relevant ADA formation
Disclosures:
David Cao indicated no relevant financial relationships.
Aaron Goffinet indicated no relevant financial relationships.
Noa Krugliak Cleveland: Johnson & Johnson – Consultant. NueroLogica – Consultant.
Russell Cohen: Abbvie – Consultant, Speakers Bureau. Bausch Health – Consultant. BMS – Consultant. Eli lilly – Consultant. Genentech – Consultant. Gilead Sciences – Consultant. Johnson & Johnson – Consultant. Pfizer – Consultant. Takeda – Consultant.
Sushila Dalal: Abbvie – Speakers Bureau.
Benjamin McDonald: Iterative Health – Consultant.
Joel Pekow: Abbvie – Stock-publicly held company(excluding mutual/index funds). CVS Health – Consultant. Eli Lilly – Stock-publicly held company(excluding mutual/index funds). Johnson and Johnson – Stock-publicly held company(excluding mutual/index funds). Pfizer – Stock-publicly held company(excluding mutual/index funds).
David Rubin: AbbVie – Advisory Committee/Board Member, Consultant, Speaker fees. Abivax SA – Consultant. Altrubio – Advisory Committee/Board Member, Consultant, Speaker feees, Stock Options. Avalo – Advisory Committee/Board Member, Consultant, Speaker fees. Bausch Health – Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speaker fees. Buhlmann Diagnostics – Advisory Committee/Board Member, Consultant, Speaker fees. Celltrion – Consultant. ClostraBio – Consultant. Connect BioPharma – Consultant. Cornerstones Health, Inc – Board of Directors membership. Douglas Pharmaceuticals – Consultant. Eli Lilly & Co. – Consultant. Foresee, Genentech (Roche) Inc. – Consultant. Image Analysis Group – Consultant. InDex Pharmaceutical – Consultant. Intouch Group – Advisory Committee/Board Member, Consultant, Speaker fees. Iterative Health – Advisory Committee/Board Member, Consultant, Speaker fees. Iterative Health – Stock Options. Janssen Pharmaceuticals – Consultant. Lilly – Advisory Committee/Board Member, Consultant, Speaker fees. Odyssey Therapeutics – Consultant. Pfizer – Advisory Committee/Board Member, Consultant, Speaker fees. Sanofi – Consultant. Takeda – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speaker fees. Throne – Consultant. Vedanta – Consultant.
Tenzin Choden indicated no relevant financial relationships.
David B. Cao, MD1, Aaron Goffinet, MD2, Noa Krugliak Cleveland, MD3, Russell D. Cohen, MD1, Sushila Dalal, MD2, Benjamin McDonald, MD, PhD3, Joel Pekow, MD3, David T. Rubin, MD4, Tenzin Choden, MD2. P1170 - Impact of Age on Anti-Drug Antibody Formation in Patients With Inflammatory Bowel Disease Treated With Tumor Necrosis Factor Inhibitors, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
