Sunday Poster Session
Category: Liver
P1714 - Acute Hepatitis During Dalbavancin Infusion: A Rare Adverse Reaction With Implications for Repeat Dosing
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
- MC
Mythri Chittilla, DO (she/her/hers)
UNC Health Blue Ridge
Ashburn, VA
Presenting Author(s)
Mythri Chittilla, DO1, Priyanka Nagdev, MD2, Claire Rinaldo, DO2, Sinclair Strange, DO2, Nidhi Garg, MD2, Emily Jacobsen, DO2, Suneel Mohammed, MD3, Rahul Sampath, MD2
1UNC Health Blue Ridge, Ashburn, VA; 2UNC Health Blue Ridge, Morganton, NC; 3UNC Health Blue Ridge, Hickory, NC
Introduction: Dalbavancin is an antibiotic approved for Gram-positive soft tissue infections, notably involving methicillin-resistant Staphylococcus aureus. It undergoes predominantly renal excretion with minimal hepatic metabolism. Clinically significant hepatotoxicity from dalbavancin is rare, occurring in fewer than 1% of treated patients. Rapid development of hepatitis soon after infusion has not been reported.
Case Description/
Methods: A 66-year-old man with a history of right rotator cuff repair developed right shoulder hardware-associated polymicrobial osteomyelitis. He underwent two debridements and a claviculectomy; cultures grew Escherichia coli, oxacillin-resistant coagulase-negative staphylococcus, and oxacillin-sensitive Staphylococcus aureus. He began IV cefazolin and received the first of two planned dalbavancin infusions (1.5 g). Two days pre-infusion, labs were within normal limits (total bilirubin 1.5 mg/dL; AST 31 U/L; ALT 21 U/L; ALP 71 U/L). Three hours after the infusion, he experienced nausea and labs revealed AST 901 U/L, ALT 316 U/L, ALP 125 U/L, total bilirubin 1.2 mg/dL. He reported three days of fatigue that self-resolved. Two days later, AST declined to 140 U/L, ALT to 106 U/L, ALP to 88 U/L, and total bilirubin to 0.8 mg/dL (Table 1). Hepatitis serologies and ANA were negative. Liver tests normalized fully by three months. He subsequently completed therapy with cefazolin and linezolid and was placed on oral cefadroxil plus doxycycline suppression in view of retained hardware.
Discussion: In pooled data from 2,120 adults across phase 1–3 studies, 17 patients (0.8%) developed post-baseline ALT elevations >3× ULN; within this group, 5 (0.2%) had ALT >10× ULN and one phase 1 subject exhibited ALT >20× ULN—each reversible on follow-up (Table 2). These enzyme spikes were seen with both the single-dose (1.5 g) and two-dose (1 g + 0.5 g one week apart) regimens, yet no study has specifically assessed outcomes when marked transaminase elevations arise after the initial 1.5 g infusion and a second 1.5 g dose is still administered. In this case likely mechanisms include immune-mediated hypersensitivity, similar to vancomycin-induced hepatotoxicity, although rate of cross-reactivity between dalbavancin and vancomycin appears to be very low to negligible. Given the growing adoption of the two-dose dalbavancin (1.5 g) regimen—we recommend obtaining liver function tests prior to administration of any second dose on a routine basis, especially if symptoms occur during the first infusion.
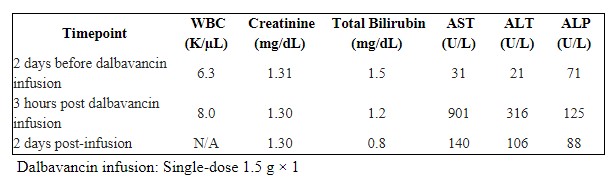
Figure: Table 1. Laboratory Values Pre-infusion and Post-infusion of Dalbavancin
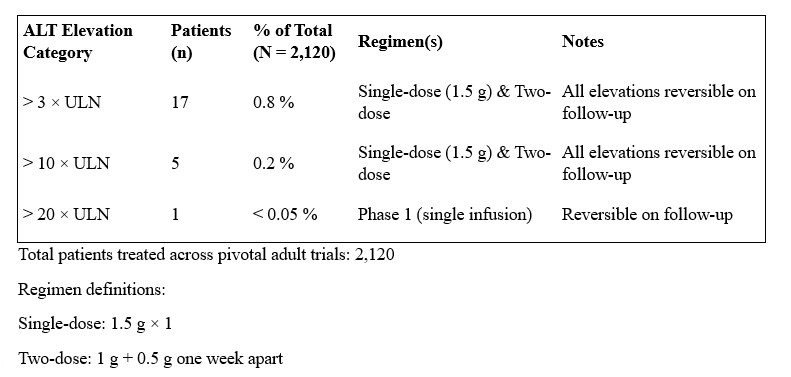
Figure: Table 2. ALT Elevation Secondary to Dalbavancin Infusion Regiments
Disclosures:
Mythri Chittilla indicated no relevant financial relationships.
Priyanka Nagdev indicated no relevant financial relationships.
Claire Rinaldo indicated no relevant financial relationships.
Sinclair Strange indicated no relevant financial relationships.
Nidhi Garg indicated no relevant financial relationships.
Emily Jacobsen indicated no relevant financial relationships.
Suneel Mohammed indicated no relevant financial relationships.
Rahul Sampath indicated no relevant financial relationships.
Mythri Chittilla, DO1, Priyanka Nagdev, MD2, Claire Rinaldo, DO2, Sinclair Strange, DO2, Nidhi Garg, MD2, Emily Jacobsen, DO2, Suneel Mohammed, MD3, Rahul Sampath, MD2. P1714 - Acute Hepatitis During Dalbavancin Infusion: A Rare Adverse Reaction With Implications for Repeat Dosing, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1UNC Health Blue Ridge, Ashburn, VA; 2UNC Health Blue Ridge, Morganton, NC; 3UNC Health Blue Ridge, Hickory, NC
Introduction: Dalbavancin is an antibiotic approved for Gram-positive soft tissue infections, notably involving methicillin-resistant Staphylococcus aureus. It undergoes predominantly renal excretion with minimal hepatic metabolism. Clinically significant hepatotoxicity from dalbavancin is rare, occurring in fewer than 1% of treated patients. Rapid development of hepatitis soon after infusion has not been reported.
Case Description/
Methods: A 66-year-old man with a history of right rotator cuff repair developed right shoulder hardware-associated polymicrobial osteomyelitis. He underwent two debridements and a claviculectomy; cultures grew Escherichia coli, oxacillin-resistant coagulase-negative staphylococcus, and oxacillin-sensitive Staphylococcus aureus. He began IV cefazolin and received the first of two planned dalbavancin infusions (1.5 g). Two days pre-infusion, labs were within normal limits (total bilirubin 1.5 mg/dL; AST 31 U/L; ALT 21 U/L; ALP 71 U/L). Three hours after the infusion, he experienced nausea and labs revealed AST 901 U/L, ALT 316 U/L, ALP 125 U/L, total bilirubin 1.2 mg/dL. He reported three days of fatigue that self-resolved. Two days later, AST declined to 140 U/L, ALT to 106 U/L, ALP to 88 U/L, and total bilirubin to 0.8 mg/dL (Table 1). Hepatitis serologies and ANA were negative. Liver tests normalized fully by three months. He subsequently completed therapy with cefazolin and linezolid and was placed on oral cefadroxil plus doxycycline suppression in view of retained hardware.
Discussion: In pooled data from 2,120 adults across phase 1–3 studies, 17 patients (0.8%) developed post-baseline ALT elevations >3× ULN; within this group, 5 (0.2%) had ALT >10× ULN and one phase 1 subject exhibited ALT >20× ULN—each reversible on follow-up (Table 2). These enzyme spikes were seen with both the single-dose (1.5 g) and two-dose (1 g + 0.5 g one week apart) regimens, yet no study has specifically assessed outcomes when marked transaminase elevations arise after the initial 1.5 g infusion and a second 1.5 g dose is still administered. In this case likely mechanisms include immune-mediated hypersensitivity, similar to vancomycin-induced hepatotoxicity, although rate of cross-reactivity between dalbavancin and vancomycin appears to be very low to negligible. Given the growing adoption of the two-dose dalbavancin (1.5 g) regimen—we recommend obtaining liver function tests prior to administration of any second dose on a routine basis, especially if symptoms occur during the first infusion.

Figure: Table 1. Laboratory Values Pre-infusion and Post-infusion of Dalbavancin

Figure: Table 2. ALT Elevation Secondary to Dalbavancin Infusion Regiments
Disclosures:
Mythri Chittilla indicated no relevant financial relationships.
Priyanka Nagdev indicated no relevant financial relationships.
Claire Rinaldo indicated no relevant financial relationships.
Sinclair Strange indicated no relevant financial relationships.
Nidhi Garg indicated no relevant financial relationships.
Emily Jacobsen indicated no relevant financial relationships.
Suneel Mohammed indicated no relevant financial relationships.
Rahul Sampath indicated no relevant financial relationships.
Mythri Chittilla, DO1, Priyanka Nagdev, MD2, Claire Rinaldo, DO2, Sinclair Strange, DO2, Nidhi Garg, MD2, Emily Jacobsen, DO2, Suneel Mohammed, MD3, Rahul Sampath, MD2. P1714 - Acute Hepatitis During Dalbavancin Infusion: A Rare Adverse Reaction With Implications for Repeat Dosing, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
