Sunday Poster Session
Category: Liver
P1786 - Probable Ashwagandha-Induced Cholestatic Hepatitis in a Healthy Young Adult: A Case Report
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Abdulwahab Ali Alqahtani, MD
King Faisal Specialist Hospital and Research Centre
Riyadh, Ar Riyad, Saudi Arabia
Presenting Author(s)
Award: ACG Presidential Poster Award
Abdulwahab Ali. Alqahtani, MD, Faisal Abaalkhail, MBBS, MM, Abdullah Alqaraawi, MD, Mohammed Alsomali, MD
King Faisal Specialist Hospital and Research Centre, Riyadh, Ar Riyad, Saudi Arabia
Introduction: Ashwagandha (Withania somnifera) is a popular adaptogenic herb in Ayurvedic medicine, often regarded as safe. However, recent reports indicate potential hepatotoxicity, including cholestatic liver injury. We present a biopsy-confirmed case of probable Ashwagandha-induced cholestatic hepatitis in a healthy young adult, highlighting the diagnostic challenges associated with herbal hepatotoxicity and the necessity of comprehensive supplement history-taking in cases of unexplained liver injury.
Case Description/
Methods: A 24-year-old male with no significant medical history presented with a two-month history of right upper quadrant pain and progressive jaundice. He began Ashwagandha supplementation 2–3 weeks prior to symptom onset. Laboratory tests revealed a cholestatic pattern of liver injury: total bilirubin 14.2 mg/dL, alkaline phosphatase (ALP) 380 U/L, aspartate aminotransferase (AST) 165 U/L, and alanine aminotransferase (ALT) 142 U/L. Extensive workup, including viral hepatitis serology, autoimmune markers, metabolic studies, abdominal ultrasound, and Magnetic resonance cholangiopancreatography, were normal. Liver biopsy revealed marked canalicular cholestasis with feathery degeneration, lobular inflammation, and mild bile duct injury, consistent with drug-induced cholestatic hepatitis. The patient received a tapering course of prednisone and ursodeoxycholic acid with discontinuation of Ashwagandha and that led to gradual normalization of liver tests and complete resolution of symptoms.
Discussion: This case underscores the potential for Ashwagandha to induce cholestatic hepatitis in young adults, reinforcing concerns regarding its hepatotoxicity. Diagnosis was supported by exclusion of other etiologies, biopsy findings, and a RUCAM score of 7. The injury is likely idiosyncratic, possibly associated with withanolide toxicity, CYP450 enzyme inhibition, or contaminants. While steroids were administered despite the absence of autoimmune features, their efficacy remains uncertain. This case highlights the importance of routine screening for herbal supplement use, improved regulation of dietary supplements, and further research into risk factors and treatment options for herb-induced liver injury.
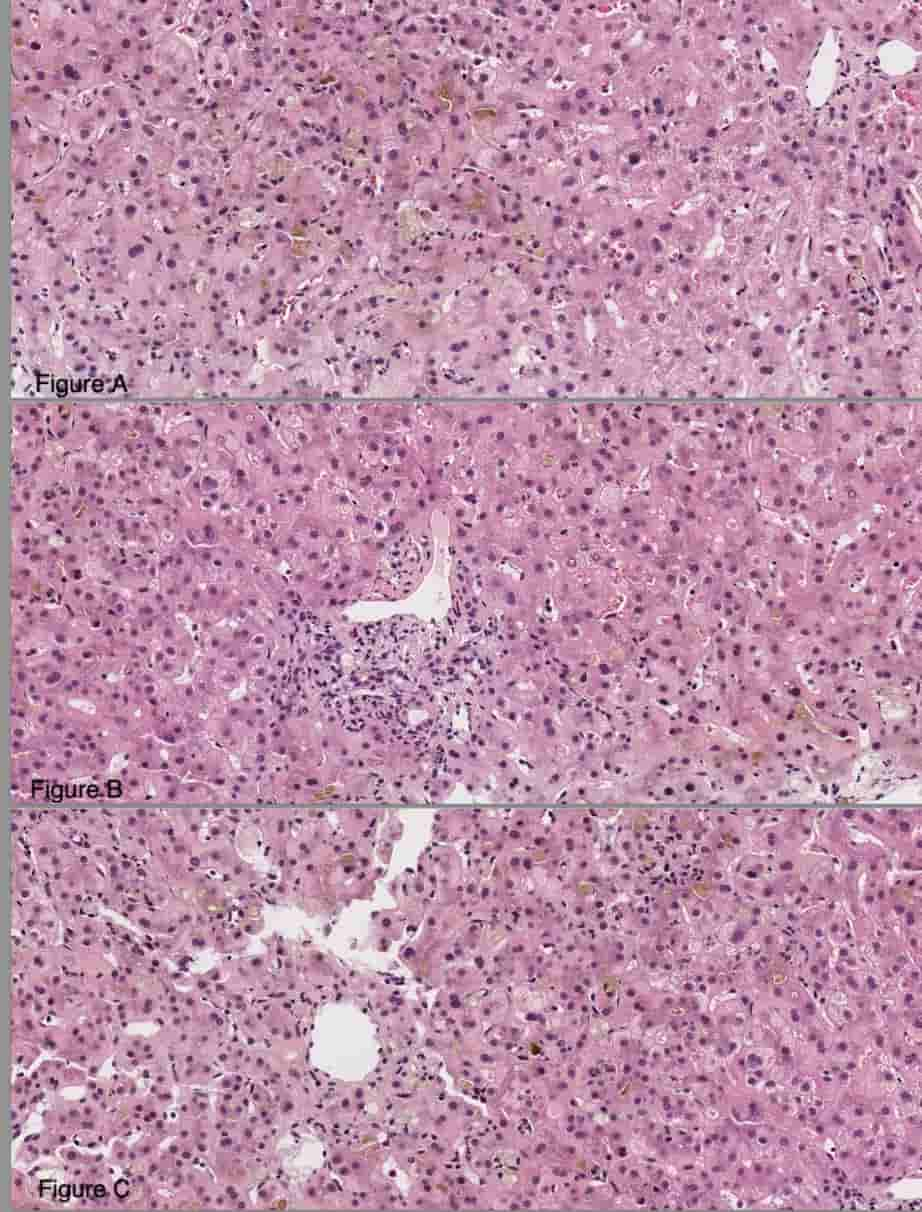
Figure: marked canalicular and hepatocellular cholestasis associated with acidophil bodies and feathery degeneration of hepatocytes (Figure 1A), mild portal inflammatory infiltrates, comprised of lymphocytes, neutrophils, rare plasma cells and eosinophils (Figure 1B). Prominent clusters of plasma cells were not seen. Foci of lobular inflammation containing lymphocytes and occasional neutrophils were identified (Figure 1C) A trichrome stain showed no significant fibrosis.
Disclosures:
Abdulwahab Alqahtani indicated no relevant financial relationships.
Faisal Abaalkhail indicated no relevant financial relationships.
Abdullah Alqaraawi indicated no relevant financial relationships.
Mohammed Alsomali indicated no relevant financial relationships.
Abdulwahab Ali. Alqahtani, MD, Faisal Abaalkhail, MBBS, MM, Abdullah Alqaraawi, MD, Mohammed Alsomali, MD. P1786 - Probable Ashwagandha-Induced Cholestatic Hepatitis in a Healthy Young Adult: A Case Report, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Abdulwahab Ali. Alqahtani, MD, Faisal Abaalkhail, MBBS, MM, Abdullah Alqaraawi, MD, Mohammed Alsomali, MD
King Faisal Specialist Hospital and Research Centre, Riyadh, Ar Riyad, Saudi Arabia
Introduction: Ashwagandha (Withania somnifera) is a popular adaptogenic herb in Ayurvedic medicine, often regarded as safe. However, recent reports indicate potential hepatotoxicity, including cholestatic liver injury. We present a biopsy-confirmed case of probable Ashwagandha-induced cholestatic hepatitis in a healthy young adult, highlighting the diagnostic challenges associated with herbal hepatotoxicity and the necessity of comprehensive supplement history-taking in cases of unexplained liver injury.
Case Description/
Methods: A 24-year-old male with no significant medical history presented with a two-month history of right upper quadrant pain and progressive jaundice. He began Ashwagandha supplementation 2–3 weeks prior to symptom onset. Laboratory tests revealed a cholestatic pattern of liver injury: total bilirubin 14.2 mg/dL, alkaline phosphatase (ALP) 380 U/L, aspartate aminotransferase (AST) 165 U/L, and alanine aminotransferase (ALT) 142 U/L. Extensive workup, including viral hepatitis serology, autoimmune markers, metabolic studies, abdominal ultrasound, and Magnetic resonance cholangiopancreatography, were normal. Liver biopsy revealed marked canalicular cholestasis with feathery degeneration, lobular inflammation, and mild bile duct injury, consistent with drug-induced cholestatic hepatitis. The patient received a tapering course of prednisone and ursodeoxycholic acid with discontinuation of Ashwagandha and that led to gradual normalization of liver tests and complete resolution of symptoms.
Discussion: This case underscores the potential for Ashwagandha to induce cholestatic hepatitis in young adults, reinforcing concerns regarding its hepatotoxicity. Diagnosis was supported by exclusion of other etiologies, biopsy findings, and a RUCAM score of 7. The injury is likely idiosyncratic, possibly associated with withanolide toxicity, CYP450 enzyme inhibition, or contaminants. While steroids were administered despite the absence of autoimmune features, their efficacy remains uncertain. This case highlights the importance of routine screening for herbal supplement use, improved regulation of dietary supplements, and further research into risk factors and treatment options for herb-induced liver injury.

Figure: marked canalicular and hepatocellular cholestasis associated with acidophil bodies and feathery degeneration of hepatocytes (Figure 1A), mild portal inflammatory infiltrates, comprised of lymphocytes, neutrophils, rare plasma cells and eosinophils (Figure 1B). Prominent clusters of plasma cells were not seen. Foci of lobular inflammation containing lymphocytes and occasional neutrophils were identified (Figure 1C) A trichrome stain showed no significant fibrosis.
Disclosures:
Abdulwahab Alqahtani indicated no relevant financial relationships.
Faisal Abaalkhail indicated no relevant financial relationships.
Abdullah Alqaraawi indicated no relevant financial relationships.
Mohammed Alsomali indicated no relevant financial relationships.
Abdulwahab Ali. Alqahtani, MD, Faisal Abaalkhail, MBBS, MM, Abdullah Alqaraawi, MD, Mohammed Alsomali, MD. P1786 - Probable Ashwagandha-Induced Cholestatic Hepatitis in a Healthy Young Adult: A Case Report, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

