Sunday Poster Session
Category: Liver
P1849 - Extraordinary Response to Odevixibat in an Adult Patient With Progressive Familial Intrahepatic Cholestasis Type 1 and Intractable Pruritus: A Case Report
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Fernando Gil Lopez, MD
Mayo Clinic
Jacksonville, FL
Presenting Author(s)
Award: ACG Presidential Poster Award
Fernando Gil Lopez, MD, Lydia Mercado, MD, Nicole Loo, MD
Mayo Clinic, Jacksonville, FL
Introduction: PFIC comprises a group of autosomal recessive disorders that share a defect in bile secretion and typically present in infancy or childhood with intrahepatic cholestasis. Clinical presentation can vary from early onset liver disease to episodic late-onset occurrence triggered by external stimuli. We report the case of a male adult who initially presented with intrahepatic cholestasis and severe pruritus at the age of 17 years old. Other primary and secondary causes were ruled out and ultimately liver biopsy and genetic testing confirmed the diagnosis of PFIC type 1. For many years, he was treated with conventional drugs for cholestasis and debilitating pruritus, without lasting, significant relief.
Case Description/
Methods: We report a case of transformational clinical improvement after treatment with odevixibat in a 27-year-old patient with refractory pruritus in the context of Progressive Familial Intrahepatic Cholestasis (PFIC) type 1. Other primary and secondary causes were ruled out and ultimately liver biopsy and genetic testing confirmed the diagnosis of PFIC type 1. He had poor response to prior therapeutic measures including cholestyramine, ursodiol, sertraline, hydroxyzine, rifampin, and topical steroids. He reported a substantial decline in his quality of life. Therefore, he was evaluated for liver transplantation at our center in March 2020 for debilitating pruritus, but was denied due to preserved liver function and low MELD score. He then started on odevixibat in November 2021 with biochemical improvement of cholestasis and dramatic, sustained resolution of pruritus. He was able to return to his normal activities achieving an excellent quality of life.
Discussion: After trying multiple treatments, this patient experienced no good relief. Odevixibat is a potent inhibitor of the apical sodium-dependent bile acid transporter, located in the terminal ileum and is fundamental in the resorption of conjugated bile acid. Most common adverse effects include fat-soluble vitamin deficiencies, abdominal pain, vomiting, and diarrhea. Self-limited diarrhea was his only reported side effect. Although proven to be safe in children, there are no clinical trials in adults for this purpose, emphasizing the relevance of our report. Regardless of the potential liver injury and cirrhosis linked to PFIC, pruritus itself can be severely debilitating, as seen in this patient. Hence, odevixibat should be considered as an option for refractory pruritus in adult patients with PFIC type 1.
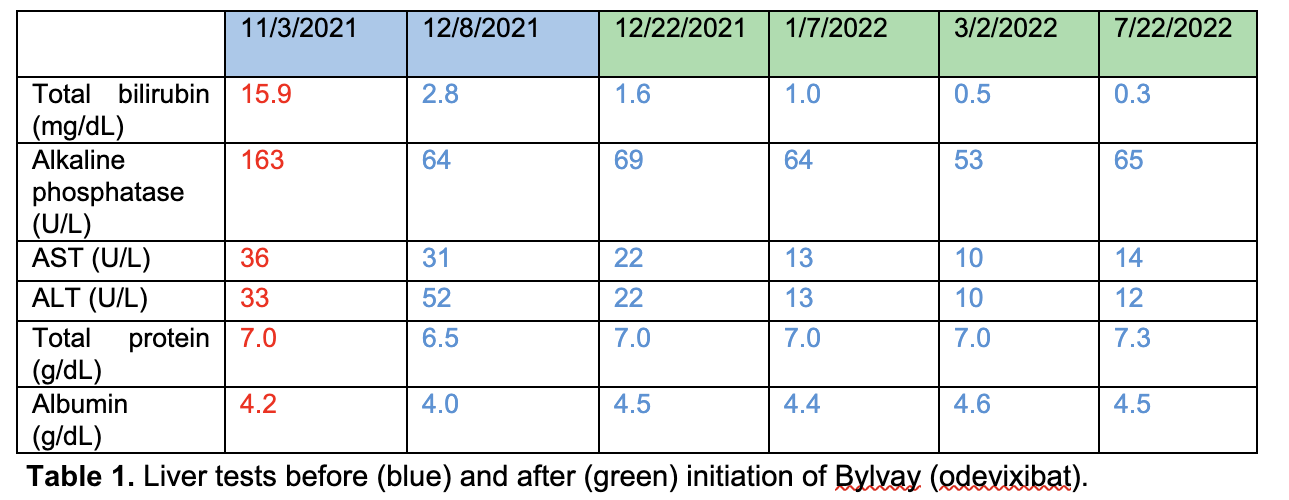
Figure: Table 1. Liver tests before (blue) and after (green) initiation of Bylvay (odevixibat).
Disclosures:
Fernando Gil Lopez indicated no relevant financial relationships.
Lydia Mercado indicated no relevant financial relationships.
Nicole Loo: Ipsen – Advisory Committee/Board Member.
Fernando Gil Lopez, MD, Lydia Mercado, MD, Nicole Loo, MD. P1849 - Extraordinary Response to Odevixibat in an Adult Patient With Progressive Familial Intrahepatic Cholestasis Type 1 and Intractable Pruritus: A Case Report, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Fernando Gil Lopez, MD, Lydia Mercado, MD, Nicole Loo, MD
Mayo Clinic, Jacksonville, FL
Introduction: PFIC comprises a group of autosomal recessive disorders that share a defect in bile secretion and typically present in infancy or childhood with intrahepatic cholestasis. Clinical presentation can vary from early onset liver disease to episodic late-onset occurrence triggered by external stimuli. We report the case of a male adult who initially presented with intrahepatic cholestasis and severe pruritus at the age of 17 years old. Other primary and secondary causes were ruled out and ultimately liver biopsy and genetic testing confirmed the diagnosis of PFIC type 1. For many years, he was treated with conventional drugs for cholestasis and debilitating pruritus, without lasting, significant relief.
Case Description/
Methods: We report a case of transformational clinical improvement after treatment with odevixibat in a 27-year-old patient with refractory pruritus in the context of Progressive Familial Intrahepatic Cholestasis (PFIC) type 1. Other primary and secondary causes were ruled out and ultimately liver biopsy and genetic testing confirmed the diagnosis of PFIC type 1. He had poor response to prior therapeutic measures including cholestyramine, ursodiol, sertraline, hydroxyzine, rifampin, and topical steroids. He reported a substantial decline in his quality of life. Therefore, he was evaluated for liver transplantation at our center in March 2020 for debilitating pruritus, but was denied due to preserved liver function and low MELD score. He then started on odevixibat in November 2021 with biochemical improvement of cholestasis and dramatic, sustained resolution of pruritus. He was able to return to his normal activities achieving an excellent quality of life.
Discussion: After trying multiple treatments, this patient experienced no good relief. Odevixibat is a potent inhibitor of the apical sodium-dependent bile acid transporter, located in the terminal ileum and is fundamental in the resorption of conjugated bile acid. Most common adverse effects include fat-soluble vitamin deficiencies, abdominal pain, vomiting, and diarrhea. Self-limited diarrhea was his only reported side effect. Although proven to be safe in children, there are no clinical trials in adults for this purpose, emphasizing the relevance of our report. Regardless of the potential liver injury and cirrhosis linked to PFIC, pruritus itself can be severely debilitating, as seen in this patient. Hence, odevixibat should be considered as an option for refractory pruritus in adult patients with PFIC type 1.

Figure: Table 1. Liver tests before (blue) and after (green) initiation of Bylvay (odevixibat).
Disclosures:
Fernando Gil Lopez indicated no relevant financial relationships.
Lydia Mercado indicated no relevant financial relationships.
Nicole Loo: Ipsen – Advisory Committee/Board Member.
Fernando Gil Lopez, MD, Lydia Mercado, MD, Nicole Loo, MD. P1849 - Extraordinary Response to Odevixibat in an Adult Patient With Progressive Familial Intrahepatic Cholestasis Type 1 and Intractable Pruritus: A Case Report, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

