Monday Poster Session
Category: Diet, Nutrition, and Obesity
P2703 - Evaluation of the Efficacy of the BARS® Device for Trans-Oral Outlet Reduction in an Ex Vivo Model Study
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- TK
Truptesh H. Kothari, MD, MS, FACG
University of Rochester Medical Center
Rochester, NY
Presenting Author(s)
Truptesh H. Kothari, MD, MS, FACG1, Smit B. Kothari, BS1, Kai Matthes, MD2, Victoria H. Howard, PA-C1
1University of Rochester Medical Center, Rochester, NY; 2Beth Israel Deaconess Medical Center, Plymouth, MA
Introduction: In patient with Roux-en-Y Gastric bypass, the restricted diameter of gastrojejunal anastomosis (GJ) limit excessive food intake; however, over time the anastomosis may increase in diameter, leading to weight recidivism. Traditional transoral outlet reduction technique with suturing device, has been used till date to decrease the anastomotic diameter to 6–8 mm with some limitations. The BARS® device approved by FDA in 2024 has been introduced as an alternative for endoscopic trans-oral outlet procedure in gastric by-pass patient with weight recidivism due to large GJ anastomosis diameter.
Methods: To evaluate the efficiency of the BARS® device in reducing the large diameter of GJ anastomosis using an ex-vivo model.
Ex-vivo pilot study.
Full-thickness porcine gastric pouches with small bowel segments were prepared to emulate an enlarged gastrojejunal anastomosis. The BARS® device was deployed at the gastrojejunal anastomosis of excised specimens. Diameter measurements were obtained pre and post deployment in both vertical and transverse orientations, and the average of these two values was used for analysis.
Results: We achieved successful anastomotic diameter reduction in all ex vivo specimens by using the BARS® device. The mean (± standard deviation [SD]) pre-intervention diameter was 23.7 ± 3.38 mm, and the mean (± SD) post-intervention diameter was 6.0 ± 3.86 mm. The difference was statistically significant (paired t-test, p = 0.002). Both vertical and transverse measurements demonstrated consistent reductions, supporting the device’s efficacy in achieving uniform clinical lumen reduction.
Discussion: In this ex-vivo pilot study, the BARS® device demonstrated a statistically significant reduction in the size of a dilated gastrojejunal anastomosis. These findings suggest that the BARS® device may be an effective alternative to traditional suturing technique for managing post-gastric bypass anastomotic dilation. Future studies will incorporate pressure assessments to further validate the efficacy of this device.
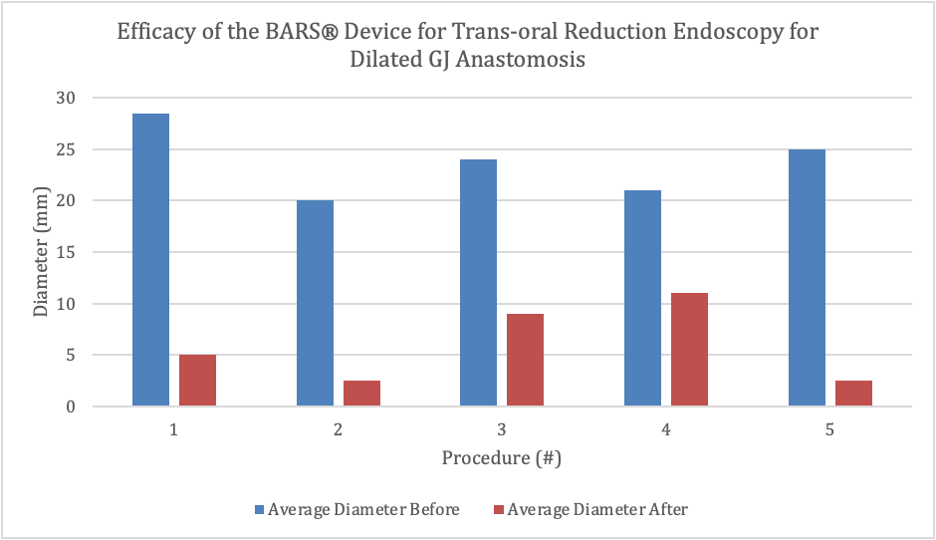
Figure: EFFICACY OF BARS DEVICE FOR TORE FOR DILATED GJ ANASTOMOSIS
Disclosures:
Truptesh Kothari indicated no relevant financial relationships.
Smit Kothari indicated no relevant financial relationships.
Kai Matthes indicated no relevant financial relationships.
Victoria Howard indicated no relevant financial relationships.
Truptesh H. Kothari, MD, MS, FACG1, Smit B. Kothari, BS1, Kai Matthes, MD2, Victoria H. Howard, PA-C1. P2703 - Evaluation of the Efficacy of the BARS® Device for Trans-Oral Outlet Reduction in an Ex Vivo Model Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Rochester Medical Center, Rochester, NY; 2Beth Israel Deaconess Medical Center, Plymouth, MA
Introduction: In patient with Roux-en-Y Gastric bypass, the restricted diameter of gastrojejunal anastomosis (GJ) limit excessive food intake; however, over time the anastomosis may increase in diameter, leading to weight recidivism. Traditional transoral outlet reduction technique with suturing device, has been used till date to decrease the anastomotic diameter to 6–8 mm with some limitations. The BARS® device approved by FDA in 2024 has been introduced as an alternative for endoscopic trans-oral outlet procedure in gastric by-pass patient with weight recidivism due to large GJ anastomosis diameter.
Methods: To evaluate the efficiency of the BARS® device in reducing the large diameter of GJ anastomosis using an ex-vivo model.
Ex-vivo pilot study.
Full-thickness porcine gastric pouches with small bowel segments were prepared to emulate an enlarged gastrojejunal anastomosis. The BARS® device was deployed at the gastrojejunal anastomosis of excised specimens. Diameter measurements were obtained pre and post deployment in both vertical and transverse orientations, and the average of these two values was used for analysis.
Results: We achieved successful anastomotic diameter reduction in all ex vivo specimens by using the BARS® device. The mean (± standard deviation [SD]) pre-intervention diameter was 23.7 ± 3.38 mm, and the mean (± SD) post-intervention diameter was 6.0 ± 3.86 mm. The difference was statistically significant (paired t-test, p = 0.002). Both vertical and transverse measurements demonstrated consistent reductions, supporting the device’s efficacy in achieving uniform clinical lumen reduction.
Discussion: In this ex-vivo pilot study, the BARS® device demonstrated a statistically significant reduction in the size of a dilated gastrojejunal anastomosis. These findings suggest that the BARS® device may be an effective alternative to traditional suturing technique for managing post-gastric bypass anastomotic dilation. Future studies will incorporate pressure assessments to further validate the efficacy of this device.

Figure: EFFICACY OF BARS DEVICE FOR TORE FOR DILATED GJ ANASTOMOSIS
Disclosures:
Truptesh Kothari indicated no relevant financial relationships.
Smit Kothari indicated no relevant financial relationships.
Kai Matthes indicated no relevant financial relationships.
Victoria Howard indicated no relevant financial relationships.
Truptesh H. Kothari, MD, MS, FACG1, Smit B. Kothari, BS1, Kai Matthes, MD2, Victoria H. Howard, PA-C1. P2703 - Evaluation of the Efficacy of the BARS® Device for Trans-Oral Outlet Reduction in an Ex Vivo Model Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
