Sunday Poster Session
Category: Stomach and Spleen
P2032 - Proportions and Outcomes of Post-Endoscopy Gastric Cancer in a US Real-World Cohort
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Himsikhar Khataniar, MD (he/him/his)
Allegheny General Hospital
Pittsburgh, PA
Presenting Author(s)
Himsikhar Khataniar, MD1, Rahul Karna, MD2, Manal Asif, MD3, Mohammad Bilal, MD, FACG4
1Allegheny General Hospital, Pittsburgh, PA; 2University of Minnesota Medical Center, Minneapolis, MN; 3Kabir Medical College, Peshawar, North-West Frontier, Pakistan; 4University of Colorado Anschutz Medical Campus, Denver, CO
Introduction: “Post-endoscopy” gastric cancer (PEGC) describes malignancy detected 6–36 months after a prior esophagogastroduodenoscopy (EGD) in which no gastric cancer was detected. While interval esophageal cancer has received well described, contemporary U.S. data on PEGC are scarce. We aimed to assess burden and 3-year clinical outcomes of PEGC compared to gastric cancers diagnosed ≤6 months after EGD (“prevalent” GC).
Methods: Using the TriNetX U.S. Collaborative Network, we identified adults (18–90 yr) carrying ICD code for GC between 2005 and 2022. A diagnosis of PEGC required a documented EGD 6–36 months before the index cancer; while a diagnosis of prevalent GC required an EGD within the preceding 6 months. Patients with prior malignancies were excluded. Propensity scores built from age, sex, race/ethnicity, cardiovascular-metabolic disease, smoking, alcohol and BMI generated a 1:1 matched cohort (n = 353). Outcomes (gastrectomy, chemotherapy, inpatient hospitalization, radiation therapy and all-cause mortality) were calculated over 3 years. Cox models yielded hazard ratios (HRs) with 95 % confidence intervals.
Results: Among 7629 GC over three years, 376 fulfilled PEGC criteria, constituting 4.9% of all diagnosed GC. Proportions of PEGC within the GC cohort stratified by sex: 4.0 % in males (191/4,760); 6.6% in females (188/2,844). By race, Whites showed a PEGC proportion of 4.6% (212/4,594), whereas the aggregated “other-race” group (Black, Asian, American Indian/Alaska Native, Native Hawaiian/Pacific Islander, and Other) had a higher proportion at 7.2% (145/2,004). Proportions of PEGC within the GC cohort stratified by ethnicity:4.2% in Hispanics (36/855); 5.7% in non-Hispanics (268/4,695). After matching, PEGC showed significantly lower chance of receiving chemotherapy (HR 0.46, 0.34–0.61), hospitalization (HR 0.61, 0.45–0.84), radiation (HR 0.36, 0.22–0.59) and mortality (HR 0.62, 0.47–0.82). Resection rates were numerically lower but not significant (HR 0.56, 0.27–1.17) in PEGC cohort. Absolute 3-year mortality was 24 % in PEGC compared to 35% in prevalent GC (risk difference –10.8 %, P = 0.002).
Discussion: Our large database study suggests that 5% of all GC are PEGC, disproportionately affecting females andracial minorities. PEGC has markedly better mortality outcome and treatment profile than prevalent GC, suggesting tumor detection at earlier stage with favorable tumor biology.
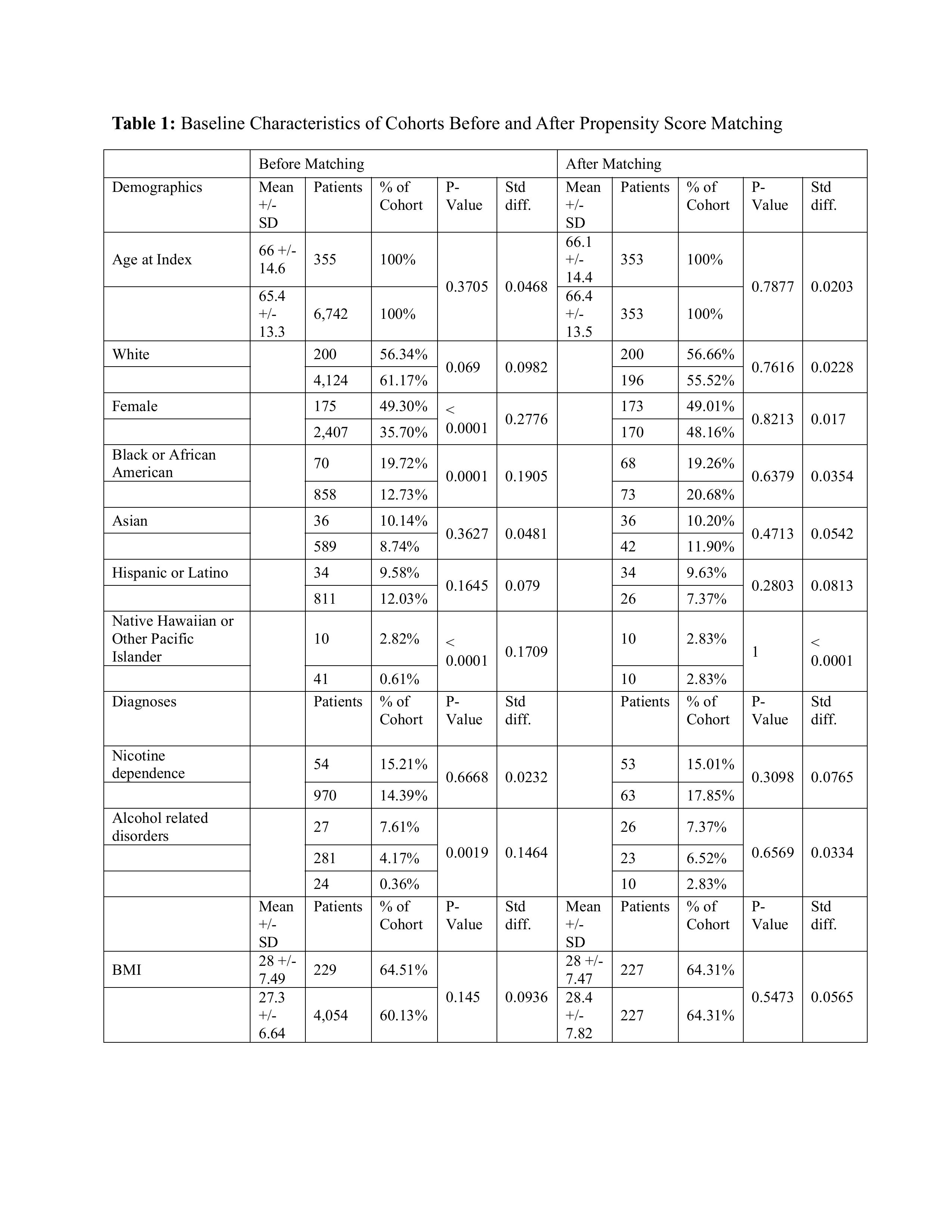
Figure: Table 1: Baseline Characteristics of Cohorts Before and After Propensity Score Matching
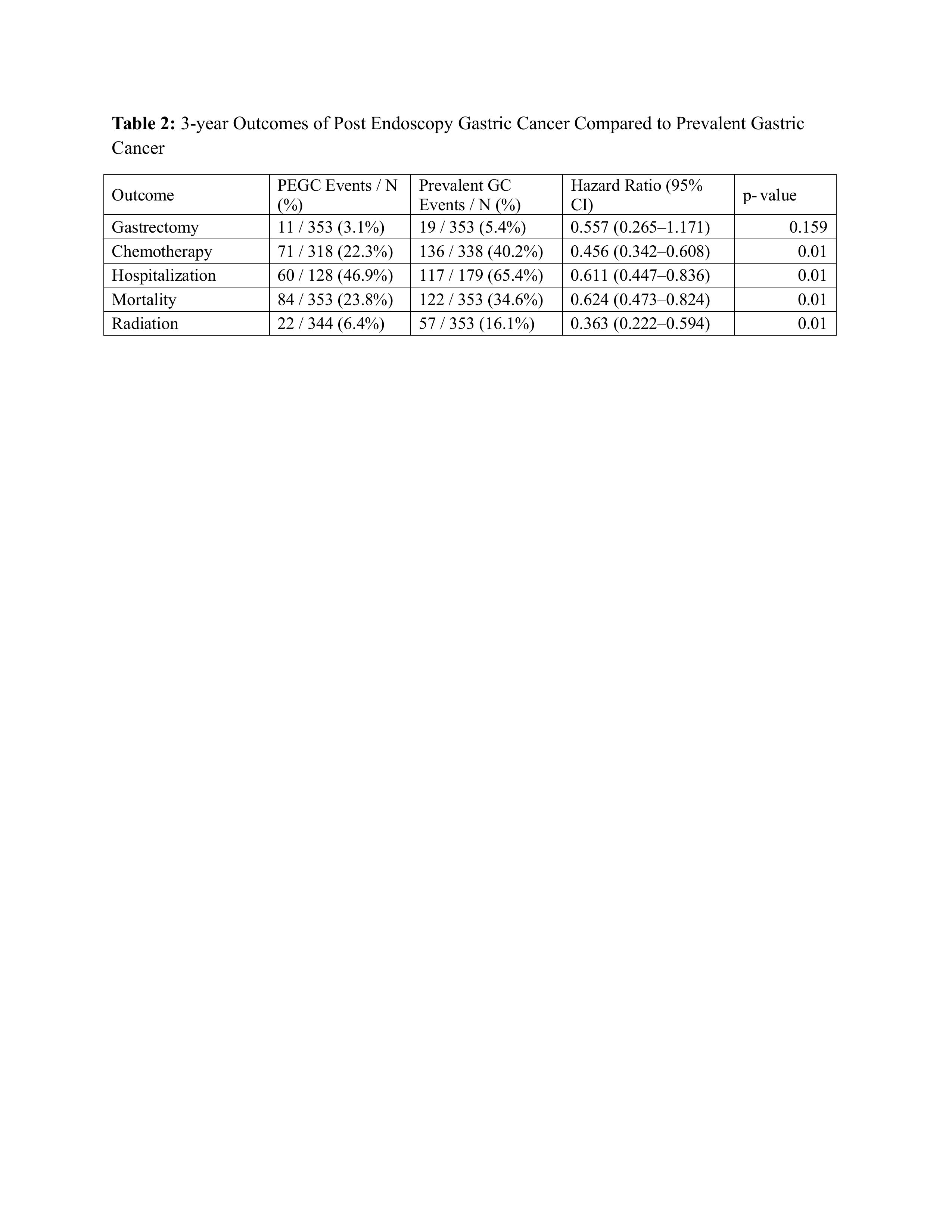
Figure: Table 2: 3-year Outcomes of Post Endoscopy Gastric Cancer Compared to Prevalent Gastric Cancer
Disclosures:
Himsikhar Khataniar indicated no relevant financial relationships.
Rahul Karna indicated no relevant financial relationships.
Manal Asif indicated no relevant financial relationships.
Mohammad Bilal: Boston Scientific – Consultant. Cook endoscopy – Paid speaker. Steris Endoscopy – Consultant.
Himsikhar Khataniar, MD1, Rahul Karna, MD2, Manal Asif, MD3, Mohammad Bilal, MD, FACG4. P2032 - Proportions and Outcomes of Post-Endoscopy Gastric Cancer in a US Real-World Cohort, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Allegheny General Hospital, Pittsburgh, PA; 2University of Minnesota Medical Center, Minneapolis, MN; 3Kabir Medical College, Peshawar, North-West Frontier, Pakistan; 4University of Colorado Anschutz Medical Campus, Denver, CO
Introduction: “Post-endoscopy” gastric cancer (PEGC) describes malignancy detected 6–36 months after a prior esophagogastroduodenoscopy (EGD) in which no gastric cancer was detected. While interval esophageal cancer has received well described, contemporary U.S. data on PEGC are scarce. We aimed to assess burden and 3-year clinical outcomes of PEGC compared to gastric cancers diagnosed ≤6 months after EGD (“prevalent” GC).
Methods: Using the TriNetX U.S. Collaborative Network, we identified adults (18–90 yr) carrying ICD code for GC between 2005 and 2022. A diagnosis of PEGC required a documented EGD 6–36 months before the index cancer; while a diagnosis of prevalent GC required an EGD within the preceding 6 months. Patients with prior malignancies were excluded. Propensity scores built from age, sex, race/ethnicity, cardiovascular-metabolic disease, smoking, alcohol and BMI generated a 1:1 matched cohort (n = 353). Outcomes (gastrectomy, chemotherapy, inpatient hospitalization, radiation therapy and all-cause mortality) were calculated over 3 years. Cox models yielded hazard ratios (HRs) with 95 % confidence intervals.
Results: Among 7629 GC over three years, 376 fulfilled PEGC criteria, constituting 4.9% of all diagnosed GC. Proportions of PEGC within the GC cohort stratified by sex: 4.0 % in males (191/4,760); 6.6% in females (188/2,844). By race, Whites showed a PEGC proportion of 4.6% (212/4,594), whereas the aggregated “other-race” group (Black, Asian, American Indian/Alaska Native, Native Hawaiian/Pacific Islander, and Other) had a higher proportion at 7.2% (145/2,004). Proportions of PEGC within the GC cohort stratified by ethnicity:4.2% in Hispanics (36/855); 5.7% in non-Hispanics (268/4,695). After matching, PEGC showed significantly lower chance of receiving chemotherapy (HR 0.46, 0.34–0.61), hospitalization (HR 0.61, 0.45–0.84), radiation (HR 0.36, 0.22–0.59) and mortality (HR 0.62, 0.47–0.82). Resection rates were numerically lower but not significant (HR 0.56, 0.27–1.17) in PEGC cohort. Absolute 3-year mortality was 24 % in PEGC compared to 35% in prevalent GC (risk difference –10.8 %, P = 0.002).
Discussion: Our large database study suggests that 5% of all GC are PEGC, disproportionately affecting females andracial minorities. PEGC has markedly better mortality outcome and treatment profile than prevalent GC, suggesting tumor detection at earlier stage with favorable tumor biology.

Figure: Table 1: Baseline Characteristics of Cohorts Before and After Propensity Score Matching

Figure: Table 2: 3-year Outcomes of Post Endoscopy Gastric Cancer Compared to Prevalent Gastric Cancer
Disclosures:
Himsikhar Khataniar indicated no relevant financial relationships.
Rahul Karna indicated no relevant financial relationships.
Manal Asif indicated no relevant financial relationships.
Mohammad Bilal: Boston Scientific – Consultant. Cook endoscopy – Paid speaker. Steris Endoscopy – Consultant.
Himsikhar Khataniar, MD1, Rahul Karna, MD2, Manal Asif, MD3, Mohammad Bilal, MD, FACG4. P2032 - Proportions and Outcomes of Post-Endoscopy Gastric Cancer in a US Real-World Cohort, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
