Monday Poster Session
Category: Diet, Nutrition, and Obesity
P2712 - Comparative Gastrointestinal Safety of Tirzepatide vs. Semaglutide: A Real-World Multicenter Analysis
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Bright Nwatamole, MBBS (he/him/his)
Vassar Brothers Medical Center - Nuvance Health
Poughkeepsie, NY
Presenting Author(s)
Bright Nwatamole, MBBS1, Nzubechukwu Stanley. Okeke, MBBS2, Adedeji Adenusi, MD, MPH3, Ibukunoluwa Oshobu, MD4
1Vassar Brothers Medical Center - Nuvance Health, Poughkeepsie, NY; 2Nuvance Health, Poughkeepsie, NY; 3One Brooklyn Health-Interfaith Medical Center, Brooklyn, NY; 4University of Missouri, Missouri, MO
Introduction: GLP-1 receptor agonists and dual GIP/GLP-1 agonists are integral to obesity treatment, but gastrointestinal (GI) adverse effects—such as gastroparesis, pancreatitis, GERD, and small intestinal bacterial overgrowth (SIBO)—remain a concern. Comparative real-world data on the GI safety of semaglutide and tirzepatide are limited.
Methods: We conducted a retrospective cohort study using TriNetX, a global federated network of de-identified electronic health records. Adults (≥18 years) prescribed semaglutide or tirzepatide were assigned to mutually exclusive cohorts, excluding those exposed to both drugs. The index date was the first prescription, with follow-up starting one day later. Patients with a prior diagnosis of any outcome were excluded from that outcome’s analysis. Propensity score matching (1:1, nearest-neighbor) was used to balance demographics and comorbidities. Primary outcomes were gastroparesis, pancreatitis, GERD, and SIBO. Risk ratios (RRs) and Kaplan-Meier analyses with hazard ratios (HRs) were used to assess incidence and time-to-event outcomes.
Results: Post-matching, each group included 280,094 patients.
Gastroparesis incidence was lower with tirzepatide (0.2%) vs. semaglutide (0.5%) [RR: 0.41; 95% CI: 0.37–0.45; p< 0.001; HR: 0.78; 95% CI: 0.70–0.86; p< 0.001].
Pancreatitis occurred in 2.1% (tirzepatide) vs. 4.2% (semaglutide) [RR: 0.51; 95% CI: 0.49–0.53; p< 0.001], but time-to-event difference was not significant (HR: 0.97; 95% CI: 0.93–1.00; p=0.07).
GERD was less common with tirzepatide (4.4%) vs. semaglutide (8.0%) [RR: 0.55; 95% CI: 0.53–0.56; p< 0.001; HR: 0.93; 95% CI: 0.90–0.95; p< 0.001].
SIBO incidence was 0.1% (tirzepatide) vs. 0.3% (semaglutide) [RR: 0.46; 95% CI: 0.40–0.52; p< 0.001], with no significant survival difference (HR: 0.96; 95% CI: 0.84–1.10; p=0.53).
Discussion: Tirzepatide was associated with a lower incidence of GI adverse events compared to semaglutide. While event rates were consistently lower, time-to-event differences were modest or non-significant for pancreatitis and SIBO. These findings suggest a potentially favorable GI safety profile for tirzepatide, supporting its use in clinical practice. Prospective studies are warranted for confirmation
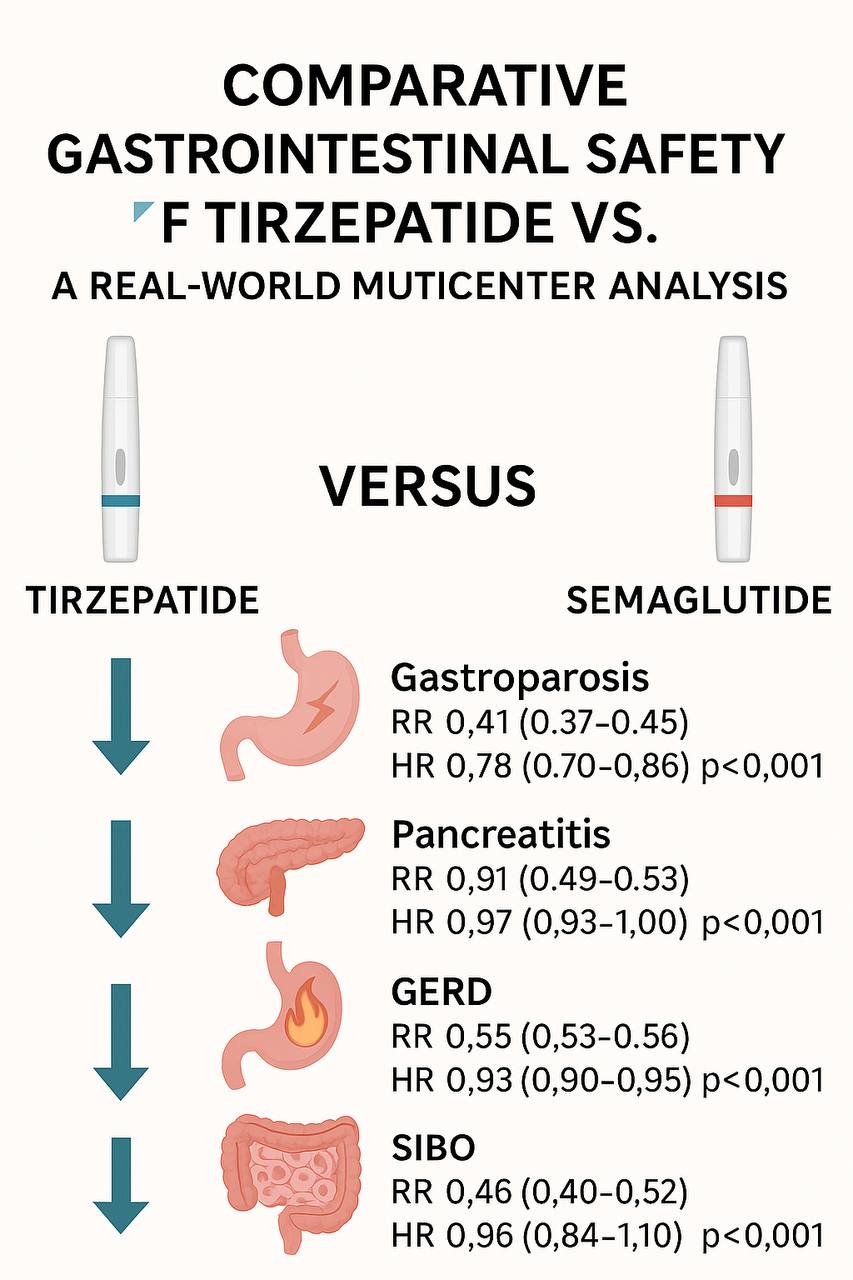
Figure: Comparative Gastrointestinal Safety of Tirzepatide Versus Semaglutide: A Multicenter Real-World Analysis
Disclosures:
Bright Nwatamole indicated no relevant financial relationships.
Nzubechukwu Okeke indicated no relevant financial relationships.
Adedeji Adenusi indicated no relevant financial relationships.
Ibukunoluwa Oshobu indicated no relevant financial relationships.
Bright Nwatamole, MBBS1, Nzubechukwu Stanley. Okeke, MBBS2, Adedeji Adenusi, MD, MPH3, Ibukunoluwa Oshobu, MD4. P2712 - Comparative Gastrointestinal Safety of Tirzepatide vs. Semaglutide: A Real-World Multicenter Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Vassar Brothers Medical Center - Nuvance Health, Poughkeepsie, NY; 2Nuvance Health, Poughkeepsie, NY; 3One Brooklyn Health-Interfaith Medical Center, Brooklyn, NY; 4University of Missouri, Missouri, MO
Introduction: GLP-1 receptor agonists and dual GIP/GLP-1 agonists are integral to obesity treatment, but gastrointestinal (GI) adverse effects—such as gastroparesis, pancreatitis, GERD, and small intestinal bacterial overgrowth (SIBO)—remain a concern. Comparative real-world data on the GI safety of semaglutide and tirzepatide are limited.
Methods: We conducted a retrospective cohort study using TriNetX, a global federated network of de-identified electronic health records. Adults (≥18 years) prescribed semaglutide or tirzepatide were assigned to mutually exclusive cohorts, excluding those exposed to both drugs. The index date was the first prescription, with follow-up starting one day later. Patients with a prior diagnosis of any outcome were excluded from that outcome’s analysis. Propensity score matching (1:1, nearest-neighbor) was used to balance demographics and comorbidities. Primary outcomes were gastroparesis, pancreatitis, GERD, and SIBO. Risk ratios (RRs) and Kaplan-Meier analyses with hazard ratios (HRs) were used to assess incidence and time-to-event outcomes.
Results: Post-matching, each group included 280,094 patients.
Gastroparesis incidence was lower with tirzepatide (0.2%) vs. semaglutide (0.5%) [RR: 0.41; 95% CI: 0.37–0.45; p< 0.001; HR: 0.78; 95% CI: 0.70–0.86; p< 0.001].
Pancreatitis occurred in 2.1% (tirzepatide) vs. 4.2% (semaglutide) [RR: 0.51; 95% CI: 0.49–0.53; p< 0.001], but time-to-event difference was not significant (HR: 0.97; 95% CI: 0.93–1.00; p=0.07).
GERD was less common with tirzepatide (4.4%) vs. semaglutide (8.0%) [RR: 0.55; 95% CI: 0.53–0.56; p< 0.001; HR: 0.93; 95% CI: 0.90–0.95; p< 0.001].
SIBO incidence was 0.1% (tirzepatide) vs. 0.3% (semaglutide) [RR: 0.46; 95% CI: 0.40–0.52; p< 0.001], with no significant survival difference (HR: 0.96; 95% CI: 0.84–1.10; p=0.53).
Discussion: Tirzepatide was associated with a lower incidence of GI adverse events compared to semaglutide. While event rates were consistently lower, time-to-event differences were modest or non-significant for pancreatitis and SIBO. These findings suggest a potentially favorable GI safety profile for tirzepatide, supporting its use in clinical practice. Prospective studies are warranted for confirmation

Figure: Comparative Gastrointestinal Safety of Tirzepatide Versus Semaglutide: A Multicenter Real-World Analysis
Disclosures:
Bright Nwatamole indicated no relevant financial relationships.
Nzubechukwu Okeke indicated no relevant financial relationships.
Adedeji Adenusi indicated no relevant financial relationships.
Ibukunoluwa Oshobu indicated no relevant financial relationships.
Bright Nwatamole, MBBS1, Nzubechukwu Stanley. Okeke, MBBS2, Adedeji Adenusi, MD, MPH3, Ibukunoluwa Oshobu, MD4. P2712 - Comparative Gastrointestinal Safety of Tirzepatide vs. Semaglutide: A Real-World Multicenter Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
