Monday Poster Session
Category: Esophagus
P2815 - Efficacy of Monthly Dupilumab in Eosinophilic Esophagitis: A Retrospective Chart Review of Patients Under 1 Year of Age or Less Than 15 Kg
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Sasha-jane Abi-Aad, MD, MSc
University of South Florida Morsani College of Medicine
Tampa, FL
Presenting Author(s)
Sasha-jane Abi-Aad, MD, MSc1, Grace Dittmar, DO1, Nicole Misner, MS, RDN1, Sharon Albers, MD1, Athanasios Tsalatsanis, PhD1, Panida Sriaroon, MD2, Racha T. Khalaf, MD, MSCS1
1University of South Florida Morsani College of Medicine, Tampa, FL; 2University of South Florida Morsani College of Medicine, St. Petersburg, FL
Introduction: Eosinophilic esophagitis (EoE) is a chronic inflammatory disease increasingly prevalent in young children, and management remains challenging as standard therapies are often insufficient to achieve remission. Dupilumab, a monoclonal antibody targeting IL-4 and IL-13 initially used for asthma and atopic dermatitis, was approved by the US Food and Drug Administration for EoE refractory to standard therapy, in 2022 for patients ≥ 12 years, and in 2024 for children ≥ 1 year and ≥ 15 kg. We conducted a retrospective chart review to explore the impact of dupilumab on EoE outcomes in patients < 1 year or < 15 kg who received this therapy for concurrent atopic dermatitis.
Methods: We conducted a retrospective single-center chart review at the University of South Florida Pediatric specialty clinics to identify individuals diagnosed with EoE (ICD-10 K20.0) at less than 1 year of age or less than 15 kg, and prescribed dupilumab (one dose every 4 weeks). Patients were included in the analysis if they had upper endoscopy (EGD) pre- and three to six months post-dupilumab initiation. Student’s t-test and chi-squared test were used (significance p < 0.05), and a patient-level plot was created to visualize individual changes in peak eosinophils along the esophageal mucosa.
Results: We identified 15 patients with EoE < 1 year or < 15 kg on dupilumab between 2021-2025. Five were excluded due to missing follow-up EGD. Among the 10 included, 60% were male, 60% identified as white and 80% as not-hispanic or latino. Mean age at dupilumab initiation was 27.8 ± 13.3 months and mean weight 12.3 ± 2.7 kg. There was a significant reduction in the mean eosinophils per high-power field (hpf) in the distal esophagus (47.8 ± 39.1 vs. 4.9 ± 8.0 p = 0.009) and mean peak eosinophils (64.1 ± 63.1 vs. 5.7 ± 8.8 p = 0.006). Individual reductions in peak eosinophils/hpf are seen in (Figure 1). However, the Eosinophilic Esophagitis Endoscopic Reference (EREF) score remained unchanged. Before dupilumab, 70% (n=7) were on dietary therapy for EoE. At follow-up, 57% of those patients (n=4) had liberalized their diet, and 100% (n=10) had a documented increase in their oral intake. Additionally, 70% had failed prior PPI and swallowed steroids (Table 1).
Discussion: Monthly dupilumab may safely and effectively induce histologic remission and improve food intake in infants and small children with EoE. Further research is needed to optimize treatment for patients with early-onset EoE and poor growth currently ineligible for dupilumab.
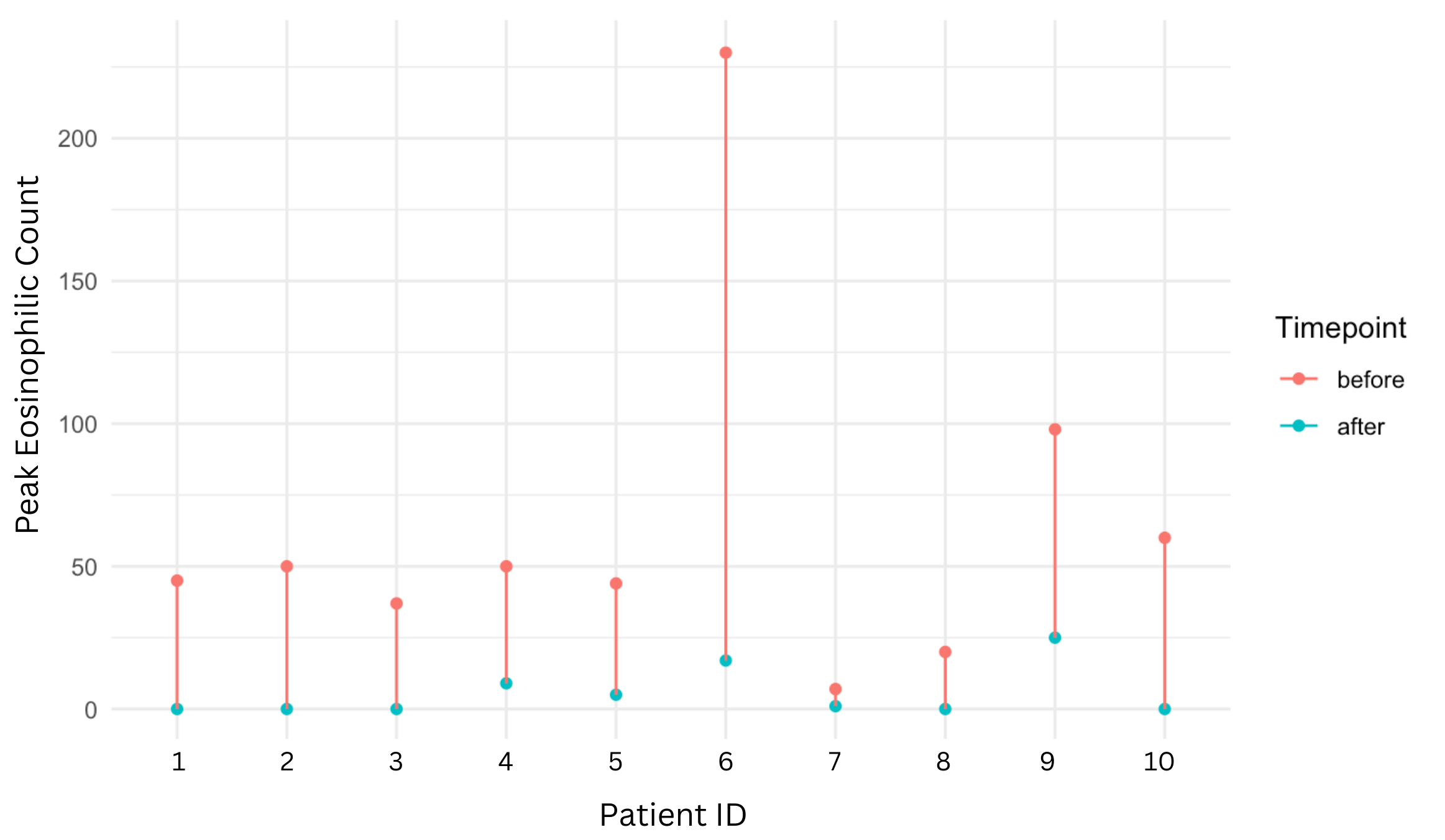
Figure: Figure 1: Individual changes in peak eosinophils per high-power field before and after dupilumab treatment in pediatric patients with eosinophilic esophagitis under 1 year of age or less than 15 kg.
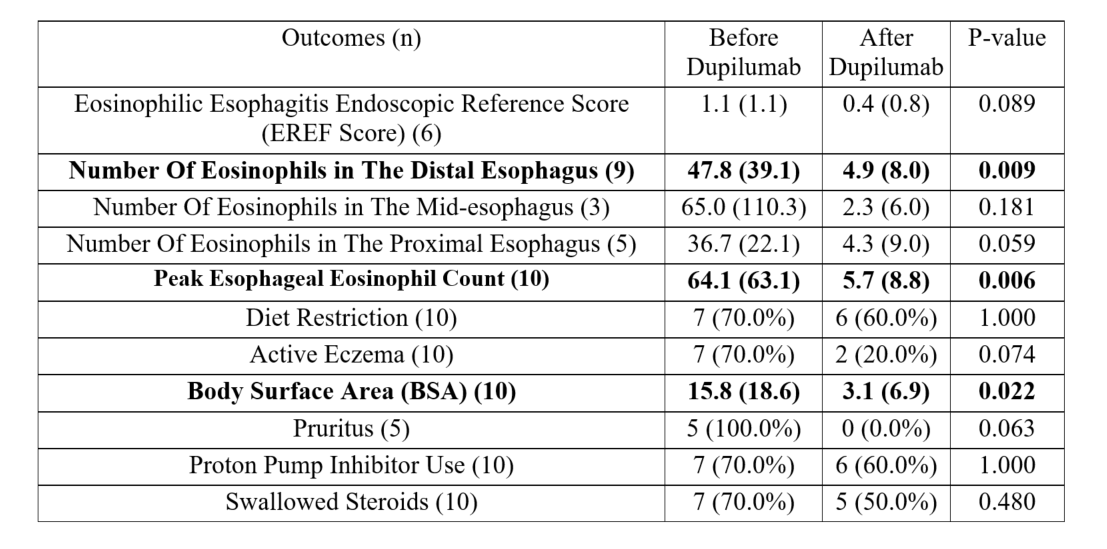
Figure: Table 1: Patient outcomes before and after 3 to 6 months of dupilumab treatment in pediatric patients with eosinophilic esophagitis under 1 year of age or less than 15 kg.
Disclosures:
Sasha-jane Abi-Aad indicated no relevant financial relationships.
Grace Dittmar indicated no relevant financial relationships.
Nicole Misner indicated no relevant financial relationships.
Sharon Albers indicated no relevant financial relationships.
Athanasios Tsalatsanis indicated no relevant financial relationships.
Panida Sriaroon: Bryn Pharma – Advisory Committee/Board Member. Genentech – Speakers Bureau. Regeneron – Principal Investigator of clinical studies sponsored by Regeneron. Takeda – Consultant.
Racha Khalaf: Sanofi – Advisory Committee/Board Member.
Sasha-jane Abi-Aad, MD, MSc1, Grace Dittmar, DO1, Nicole Misner, MS, RDN1, Sharon Albers, MD1, Athanasios Tsalatsanis, PhD1, Panida Sriaroon, MD2, Racha T. Khalaf, MD, MSCS1. P2815 - Efficacy of Monthly Dupilumab in Eosinophilic Esophagitis: A Retrospective Chart Review of Patients Under 1 Year of Age or Less Than 15 Kg, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of South Florida Morsani College of Medicine, Tampa, FL; 2University of South Florida Morsani College of Medicine, St. Petersburg, FL
Introduction: Eosinophilic esophagitis (EoE) is a chronic inflammatory disease increasingly prevalent in young children, and management remains challenging as standard therapies are often insufficient to achieve remission. Dupilumab, a monoclonal antibody targeting IL-4 and IL-13 initially used for asthma and atopic dermatitis, was approved by the US Food and Drug Administration for EoE refractory to standard therapy, in 2022 for patients ≥ 12 years, and in 2024 for children ≥ 1 year and ≥ 15 kg. We conducted a retrospective chart review to explore the impact of dupilumab on EoE outcomes in patients < 1 year or < 15 kg who received this therapy for concurrent atopic dermatitis.
Methods: We conducted a retrospective single-center chart review at the University of South Florida Pediatric specialty clinics to identify individuals diagnosed with EoE (ICD-10 K20.0) at less than 1 year of age or less than 15 kg, and prescribed dupilumab (one dose every 4 weeks). Patients were included in the analysis if they had upper endoscopy (EGD) pre- and three to six months post-dupilumab initiation. Student’s t-test and chi-squared test were used (significance p < 0.05), and a patient-level plot was created to visualize individual changes in peak eosinophils along the esophageal mucosa.
Results: We identified 15 patients with EoE < 1 year or < 15 kg on dupilumab between 2021-2025. Five were excluded due to missing follow-up EGD. Among the 10 included, 60% were male, 60% identified as white and 80% as not-hispanic or latino. Mean age at dupilumab initiation was 27.8 ± 13.3 months and mean weight 12.3 ± 2.7 kg. There was a significant reduction in the mean eosinophils per high-power field (hpf) in the distal esophagus (47.8 ± 39.1 vs. 4.9 ± 8.0 p = 0.009) and mean peak eosinophils (64.1 ± 63.1 vs. 5.7 ± 8.8 p = 0.006). Individual reductions in peak eosinophils/hpf are seen in (Figure 1). However, the Eosinophilic Esophagitis Endoscopic Reference (EREF) score remained unchanged. Before dupilumab, 70% (n=7) were on dietary therapy for EoE. At follow-up, 57% of those patients (n=4) had liberalized their diet, and 100% (n=10) had a documented increase in their oral intake. Additionally, 70% had failed prior PPI and swallowed steroids (Table 1).
Discussion: Monthly dupilumab may safely and effectively induce histologic remission and improve food intake in infants and small children with EoE. Further research is needed to optimize treatment for patients with early-onset EoE and poor growth currently ineligible for dupilumab.

Figure: Figure 1: Individual changes in peak eosinophils per high-power field before and after dupilumab treatment in pediatric patients with eosinophilic esophagitis under 1 year of age or less than 15 kg.

Figure: Table 1: Patient outcomes before and after 3 to 6 months of dupilumab treatment in pediatric patients with eosinophilic esophagitis under 1 year of age or less than 15 kg.
Disclosures:
Sasha-jane Abi-Aad indicated no relevant financial relationships.
Grace Dittmar indicated no relevant financial relationships.
Nicole Misner indicated no relevant financial relationships.
Sharon Albers indicated no relevant financial relationships.
Athanasios Tsalatsanis indicated no relevant financial relationships.
Panida Sriaroon: Bryn Pharma – Advisory Committee/Board Member. Genentech – Speakers Bureau. Regeneron – Principal Investigator of clinical studies sponsored by Regeneron. Takeda – Consultant.
Racha Khalaf: Sanofi – Advisory Committee/Board Member.
Sasha-jane Abi-Aad, MD, MSc1, Grace Dittmar, DO1, Nicole Misner, MS, RDN1, Sharon Albers, MD1, Athanasios Tsalatsanis, PhD1, Panida Sriaroon, MD2, Racha T. Khalaf, MD, MSCS1. P2815 - Efficacy of Monthly Dupilumab in Eosinophilic Esophagitis: A Retrospective Chart Review of Patients Under 1 Year of Age or Less Than 15 Kg, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

