Monday Poster Session
Category: Esophagus
P2812 - Sustained Success of Proton Pump Inhibitor De-Prescription: A 1-Year Follow-Up From an Internal Medicine Resident Clinic Experience
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- JL
James Lee, MD
Stony Brook Medicine
Stony Brook, NY
Presenting Author(s)
James Lee, MD1, Rahul Tripathi, MD1, David Stein, MD1, Michael Jorgensen, MD1, Nimra Hameed, MD1, Daniel Jamorabo, MD2, Lisa Fisher, MD3
1Stony Brook Medicine, Stony Brook, NY; 2Northwell Health, Forest Hills, NY; 3Stony Brook University Hospital, Northport, NY
Introduction: Proton pump inhibitors (PPIs) are commonly prescribed for prolonged durations, often without a clear ongoing indication, contributing to unnecessary polypharmacy and risk of adverse outcomes. In our resident-run primary care clinic, a quality improvement initiative using a VA-guided deprescription protocol identified 108 patients on PPIs. Of these, 85 (79%) were appropriately prescribed, while 23 (21%) were eligible for dose reduction or discontinuation. Following intervention, 20 of the 23 patients (87%) were successfully deprescribed or dose reduced. This study assesses the long-term durability and safety of deprescription over a 12-month follow-up period.
Methods: We conducted a retrospective chart review of 20 out of 108 patients who met criteria and underwent PPI dose reduction or discontinuation approximately 12 months prior (Figure 1) . Using the electronic medical record (EMR), we assessed medication reconciliation, progress notes, and prescription refill histories to determine whether patients remained off PPIs or maintained a reduced dose at 12 months. Sustained deprescription was defined as continued absence of PPI therapy or maintenance of a reduced regimen without escalation back to the prior dose.
Results: Of the 20 patients initially deprescribed or dose-reduced from PPI therapy, 15 (75%) patients remained off PPI or on a reduced dose without escalation, 4 (20%) resumed PPI or resumed to previous dose PPI, and 1 patient passed away during the follow up period. Among those who remained off or on reduced therapy, symptom control was generally adequate; several reported no reflux symptoms, while others noted mild, intermittent symptoms (≤2x/week), which were effectively managed with as-needed famotidine. No adverse outcomes such as upper gastrointestinal bleeding, hospitalization relating to upper gastrointestinal, or escalation beyond baseline PPI dose were documented.
Discussion: At 12-month follow-up, 75% of patients who underwent PPI deprescription or dose reduction remained off therapy or maintained a lower dose without escalation, with minimal symptom recurrence and no serious adverse events. Routine medication review and appropriate deprescription efforts can help reduce pill burden and improve guideline-concordant prescribing without compromising patient outcomes. Further quality improvement initiatives are needed to support appropriate PPI deprescribing and to educate patients on safe and effective tapering strategies.
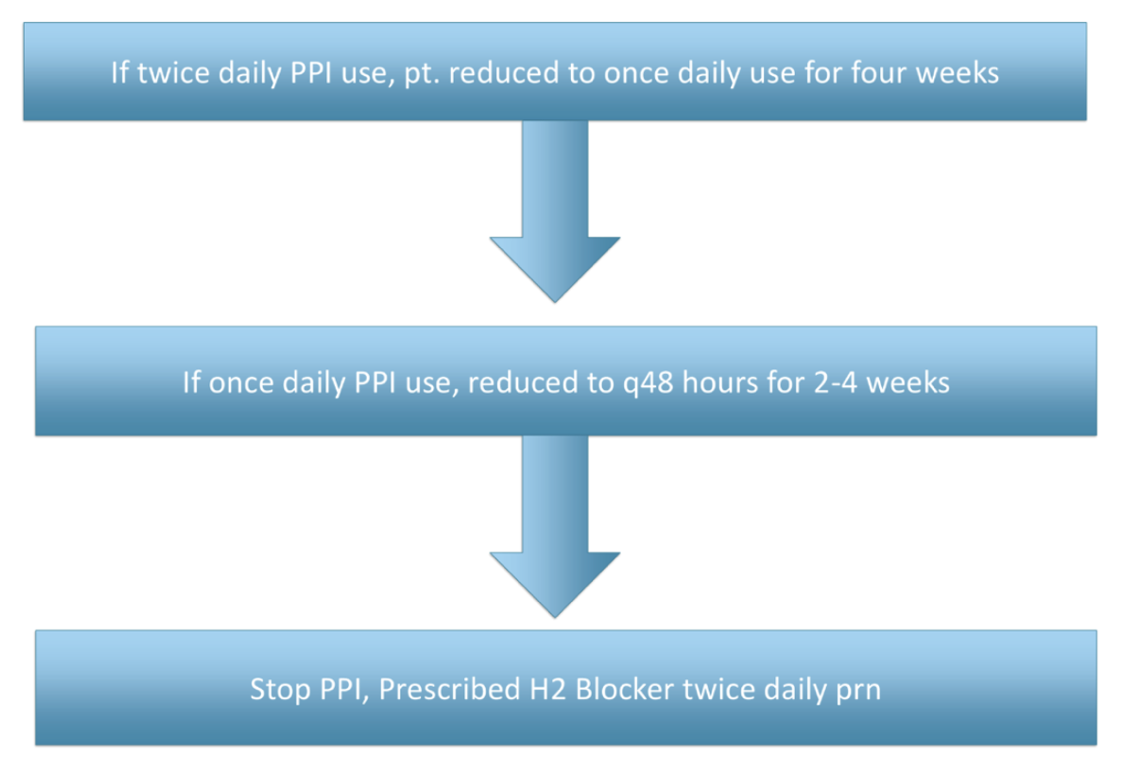
Figure: Figure 1. PPI Tapering Schedule
Disclosures:
James Lee indicated no relevant financial relationships.
Rahul Tripathi indicated no relevant financial relationships.
David Stein indicated no relevant financial relationships.
Michael Jorgensen indicated no relevant financial relationships.
Nimra Hameed indicated no relevant financial relationships.
Daniel Jamorabo indicated no relevant financial relationships.
Lisa Fisher indicated no relevant financial relationships.
James Lee, MD1, Rahul Tripathi, MD1, David Stein, MD1, Michael Jorgensen, MD1, Nimra Hameed, MD1, Daniel Jamorabo, MD2, Lisa Fisher, MD3. P2812 - Sustained Success of Proton Pump Inhibitor De-Prescription: A 1-Year Follow-Up From an Internal Medicine Resident Clinic Experience, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Stony Brook Medicine, Stony Brook, NY; 2Northwell Health, Forest Hills, NY; 3Stony Brook University Hospital, Northport, NY
Introduction: Proton pump inhibitors (PPIs) are commonly prescribed for prolonged durations, often without a clear ongoing indication, contributing to unnecessary polypharmacy and risk of adverse outcomes. In our resident-run primary care clinic, a quality improvement initiative using a VA-guided deprescription protocol identified 108 patients on PPIs. Of these, 85 (79%) were appropriately prescribed, while 23 (21%) were eligible for dose reduction or discontinuation. Following intervention, 20 of the 23 patients (87%) were successfully deprescribed or dose reduced. This study assesses the long-term durability and safety of deprescription over a 12-month follow-up period.
Methods: We conducted a retrospective chart review of 20 out of 108 patients who met criteria and underwent PPI dose reduction or discontinuation approximately 12 months prior (Figure 1) . Using the electronic medical record (EMR), we assessed medication reconciliation, progress notes, and prescription refill histories to determine whether patients remained off PPIs or maintained a reduced dose at 12 months. Sustained deprescription was defined as continued absence of PPI therapy or maintenance of a reduced regimen without escalation back to the prior dose.
Results: Of the 20 patients initially deprescribed or dose-reduced from PPI therapy, 15 (75%) patients remained off PPI or on a reduced dose without escalation, 4 (20%) resumed PPI or resumed to previous dose PPI, and 1 patient passed away during the follow up period. Among those who remained off or on reduced therapy, symptom control was generally adequate; several reported no reflux symptoms, while others noted mild, intermittent symptoms (≤2x/week), which were effectively managed with as-needed famotidine. No adverse outcomes such as upper gastrointestinal bleeding, hospitalization relating to upper gastrointestinal, or escalation beyond baseline PPI dose were documented.
Discussion: At 12-month follow-up, 75% of patients who underwent PPI deprescription or dose reduction remained off therapy or maintained a lower dose without escalation, with minimal symptom recurrence and no serious adverse events. Routine medication review and appropriate deprescription efforts can help reduce pill burden and improve guideline-concordant prescribing without compromising patient outcomes. Further quality improvement initiatives are needed to support appropriate PPI deprescribing and to educate patients on safe and effective tapering strategies.

Figure: Figure 1. PPI Tapering Schedule
Disclosures:
James Lee indicated no relevant financial relationships.
Rahul Tripathi indicated no relevant financial relationships.
David Stein indicated no relevant financial relationships.
Michael Jorgensen indicated no relevant financial relationships.
Nimra Hameed indicated no relevant financial relationships.
Daniel Jamorabo indicated no relevant financial relationships.
Lisa Fisher indicated no relevant financial relationships.
James Lee, MD1, Rahul Tripathi, MD1, David Stein, MD1, Michael Jorgensen, MD1, Nimra Hameed, MD1, Daniel Jamorabo, MD2, Lisa Fisher, MD3. P2812 - Sustained Success of Proton Pump Inhibitor De-Prescription: A 1-Year Follow-Up From an Internal Medicine Resident Clinic Experience, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
