Monday Poster Session
Category: Esophagus
P2903 - A Rare Case of Immune Checkpoint Inhibitor Esophagitis Due to Pembrolizumab
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Marina Khan, MD (she/her/hers)
University of Toledo Medical Center
Toledo, OH
Presenting Author(s)
Marina Khan, MD1, Faisal S. Shariff, MD2, Ateeb Parvez, MBBS3, Zarmina Khan, DMD4, Fnu Sameeullah, MD5, Danae Hamouda, MD6
1University of Toledo Medical Center, Toledo, OH; 2University of Toledo Medical Center, Sylvania, OH; 3St. Elizabeth's Medical Center, Boston University School of Medicine, Quincy, MA; 4UF Health Jacksonville, Jacksonville, FL; 5Boston Medical Center, Boston University School of Medicine, Dorchester, MA; 6University of Toledo Medical Center, Hematology and Oncology fellowship Program Director, Toledo, OH
Introduction: Immune-mediated esophagitis and ulcerative mucositis represent rare adverse effects of pembrolizumab therapy. We describe a case of grade 3/4 pembrolizumab-induced esophagitis and mucositis in a patient receiving treatment for stage IIA breast adenocarcinoma.
Case Description/
Methods: A 72-year-old female with triple-negative, poorly differentiated stage IIA breast adenocarcinoma achieved complete response to neoadjuvant therapy (doxorubicin, cyclophosphamide, and pembrolizumab). During adjuvant pembrolizumab treatment, she developed severe odynophagia. Physical examination revealed posterior oropharyngeal erythema and lingual mucositis. Her symptoms proved refractory to empiric therapies including azithromycin, nystatin, fluconazole, micafungin, and saline gargles. Initial infectious workup was negative, but neck CT demonstrated mucosal enhancement of the epiglottis and aryepiglottic folds suggestive of mucositis. Flexible laryngoscopy confirmed ulcerative mucositis with concomitant laryngopharyngitis.
Pembrolizumab was discontinued, and she was initiated on prednisone 1 mg/kg, achieving significant pain improvement within one week. However, symptom recurrence occurred during steroid taper after five weeks. Subsequent EGD revealed diffuse gastric hyperemia, edema, and antral ulcers, with cultures positive for Candida albicans (HSV and CMV negative). Treatment with fluconazole and pantoprazole was initiated. Repeat endoscopy demonstrated persistent pharyngo-esophagitis with mid-esophageal ulceration, confirming chronic active immune-mediated esophagitis. Escalation to prednisone 1.5 mg/kg daily with continued PPI therapy ultimately resulted in complete symptom resolution.
Discussion: Pembrolizumab, a PD-1 inhibitor monoclonal antibody, boosts antitumor immunity by blocking PD-1/PD-L1/2 interactions. This immune activation, though effective, can cause immune-related adverse events (irAEs) across organ systems. GI irAEs more often affect the lower tract (e.g., colitis), especially with CTLA-4 inhibitors, but PD-1/PD-L1 therapies show distinct upper GI patterns. These usually appear later (3–6 months post-treatment) and primarily involve the stomach. Only about 25 cases of ICI-related esophagitis are reported. Management includes acid suppression (PPIs, H2 blockers), mucosal protectants (sucralfate), and corticosteroids for moderate to severe cases. This case highlights the need for clinician awareness of rare upper GI irAEs, given their delayed onset, significant morbidity and complex treatment.
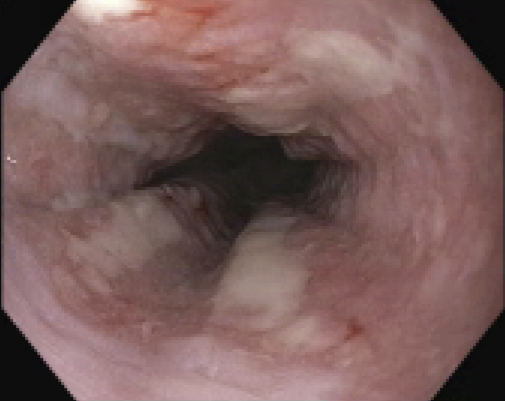
Figure: Multiple linear esophageal ulcers were found spanning 25-32 cm from the incisors at the mid esophagus
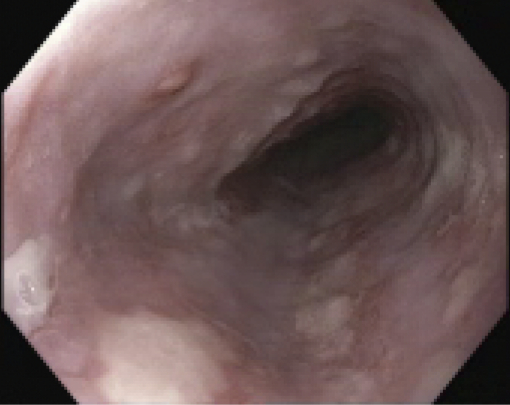
Figure: Multiple superficial esophageal ulcers with significant friability in the mid esophagus
Disclosures:
Marina Khan indicated no relevant financial relationships.
Faisal Shariff indicated no relevant financial relationships.
Ateeb Parvez indicated no relevant financial relationships.
Zarmina Khan indicated no relevant financial relationships.
Fnu Sameeullah indicated no relevant financial relationships.
Danae Hamouda indicated no relevant financial relationships.
Marina Khan, MD1, Faisal S. Shariff, MD2, Ateeb Parvez, MBBS3, Zarmina Khan, DMD4, Fnu Sameeullah, MD5, Danae Hamouda, MD6. P2903 - A Rare Case of Immune Checkpoint Inhibitor Esophagitis Due to Pembrolizumab, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Toledo Medical Center, Toledo, OH; 2University of Toledo Medical Center, Sylvania, OH; 3St. Elizabeth's Medical Center, Boston University School of Medicine, Quincy, MA; 4UF Health Jacksonville, Jacksonville, FL; 5Boston Medical Center, Boston University School of Medicine, Dorchester, MA; 6University of Toledo Medical Center, Hematology and Oncology fellowship Program Director, Toledo, OH
Introduction: Immune-mediated esophagitis and ulcerative mucositis represent rare adverse effects of pembrolizumab therapy. We describe a case of grade 3/4 pembrolizumab-induced esophagitis and mucositis in a patient receiving treatment for stage IIA breast adenocarcinoma.
Case Description/
Methods: A 72-year-old female with triple-negative, poorly differentiated stage IIA breast adenocarcinoma achieved complete response to neoadjuvant therapy (doxorubicin, cyclophosphamide, and pembrolizumab). During adjuvant pembrolizumab treatment, she developed severe odynophagia. Physical examination revealed posterior oropharyngeal erythema and lingual mucositis. Her symptoms proved refractory to empiric therapies including azithromycin, nystatin, fluconazole, micafungin, and saline gargles. Initial infectious workup was negative, but neck CT demonstrated mucosal enhancement of the epiglottis and aryepiglottic folds suggestive of mucositis. Flexible laryngoscopy confirmed ulcerative mucositis with concomitant laryngopharyngitis.
Pembrolizumab was discontinued, and she was initiated on prednisone 1 mg/kg, achieving significant pain improvement within one week. However, symptom recurrence occurred during steroid taper after five weeks. Subsequent EGD revealed diffuse gastric hyperemia, edema, and antral ulcers, with cultures positive for Candida albicans (HSV and CMV negative). Treatment with fluconazole and pantoprazole was initiated. Repeat endoscopy demonstrated persistent pharyngo-esophagitis with mid-esophageal ulceration, confirming chronic active immune-mediated esophagitis. Escalation to prednisone 1.5 mg/kg daily with continued PPI therapy ultimately resulted in complete symptom resolution.
Discussion: Pembrolizumab, a PD-1 inhibitor monoclonal antibody, boosts antitumor immunity by blocking PD-1/PD-L1/2 interactions. This immune activation, though effective, can cause immune-related adverse events (irAEs) across organ systems. GI irAEs more often affect the lower tract (e.g., colitis), especially with CTLA-4 inhibitors, but PD-1/PD-L1 therapies show distinct upper GI patterns. These usually appear later (3–6 months post-treatment) and primarily involve the stomach. Only about 25 cases of ICI-related esophagitis are reported. Management includes acid suppression (PPIs, H2 blockers), mucosal protectants (sucralfate), and corticosteroids for moderate to severe cases. This case highlights the need for clinician awareness of rare upper GI irAEs, given their delayed onset, significant morbidity and complex treatment.

Figure: Multiple linear esophageal ulcers were found spanning 25-32 cm from the incisors at the mid esophagus

Figure: Multiple superficial esophageal ulcers with significant friability in the mid esophagus
Disclosures:
Marina Khan indicated no relevant financial relationships.
Faisal Shariff indicated no relevant financial relationships.
Ateeb Parvez indicated no relevant financial relationships.
Zarmina Khan indicated no relevant financial relationships.
Fnu Sameeullah indicated no relevant financial relationships.
Danae Hamouda indicated no relevant financial relationships.
Marina Khan, MD1, Faisal S. Shariff, MD2, Ateeb Parvez, MBBS3, Zarmina Khan, DMD4, Fnu Sameeullah, MD5, Danae Hamouda, MD6. P2903 - A Rare Case of Immune Checkpoint Inhibitor Esophagitis Due to Pembrolizumab, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
