Monday Poster Session
Category: General Endoscopy
P3039 - Biopsy Beyond the Surface: A Challenging Case of Refractory Diarrhea Secondary to CAR-T Therapy
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Bara Abujaber, MBBS (he/him/his)
MedStar Georgetown/Washington Hospital Center
Washington, DC
Presenting Author(s)
Bara Abujaber, MBBS1, Nour Abu Irhayem, MBBS2, Joseph Alukal, MD3, Mark Mattar, MD3
1MedStar Georgetown/Washington Hospital Center, Washington, DC; 2MedStar Georgetown/ Washington Hospital Center, Washington, DC; 3MedStar Georgetown University Hospital, Washington, DC
Introduction: Chronic diarrhea in immunocompromised patients presents a broad range of differentials. In recipients of chimeric antigen receptor T-cell (CAR-T) therapy, delayed-onset diarrhea is often attributed to immune effector cell (IEC)–associated enterocolitis and typically responds to steroid treatment. However, persistent, steroid-refractory diarrhea may represent a rare but emerging complication: CAR-T–associated GI lymphoproliferative (LP) disorders.
Case Description/
Methods: A 70-year-old female with multiple myeloma presented with chronic, non-bloody, mucoid diarrhea ( >5 episodes/day) seven months after Cilta-cel CAR-T therapy. Her workup was negative for inflammatory, autoimmune, and common infectious causes. CMV DNA PCR was mildly elevated (555 copies/mL) and was refractory to ganciclovir and empiric antibiotics. She was later treated with steroids for suspected Graft Versus Host Disease, but diarrhea persisted. She had normal mucosa on upper and lower endoscopies, but random biopsies revealed diffuse granzyme B-positive CD8+ T-cell infiltration in the colon, duodenum, and esophagus. Sequencing verified CAR-T construct integration and clonal proliferation consistent with indolent CD8+ CAR-T cell lymphoma. She was started on IV cyclosporine 2.5 mg/kg for 14 days with symptom resolution. Although she recovered from a GI perspective, she developed influenza pneumonia and expired.
Discussion: This case highlights an extremely rare but increasingly recognized GI complication following CAR-T therapy that warrants timely diagnosis and intervention. IEC-associated enterocolitis occurs in ~1.2% of CAR-T recipients, but CAR-T–induced T-cell lymphoma following Cilta-cel has only been reported twice. The gut may act as a reservoir for clonal CAR-T expansion, with potential for systemic dissemination. Persistent diarrhea unresponsive to corticosteroids should prompt bidirectional endoscopy with biopsy. Cyclosporine is thought to treat this condition by uniquely targeting persistent engineered CAR-T cells by inhibiting IL-2-mediated mucosal proliferation and is administered at 3–5 mg/kg/day with goal troughs between 150–250 ng/mL. Given the similarity between CAR-T cell lymphoma and IBD, further research is needed on the potential role of biologics in treating this rare disease entity.
References:
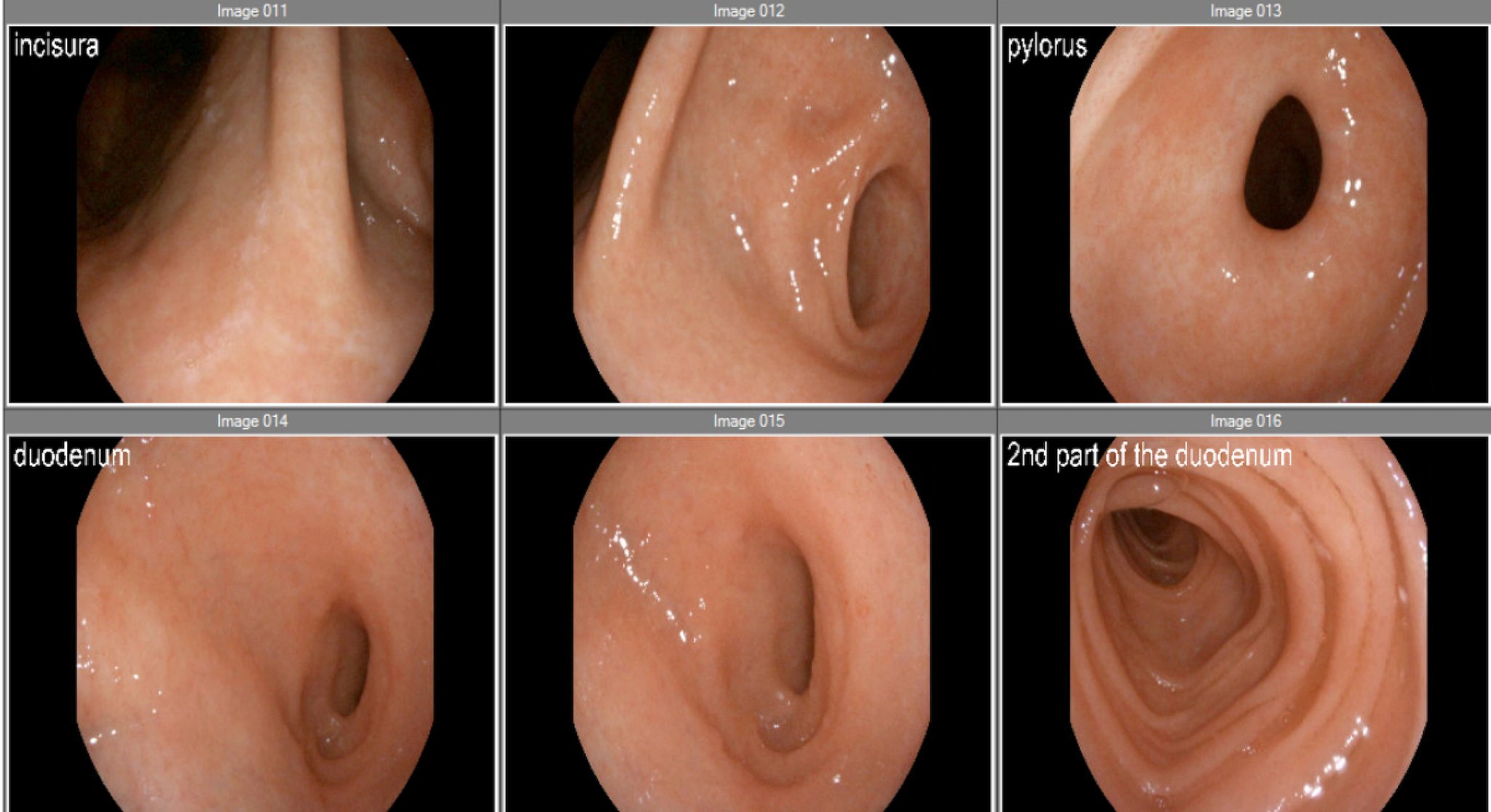
Figure: Figure 1. Endoscopic images from esophagogastroduodenoscopy (EGD) demonstrating normal-appearing mucosa in the pylorus and duodenum despite histologic evidence of CD8+ CAR-T cell infiltration on biopsy.
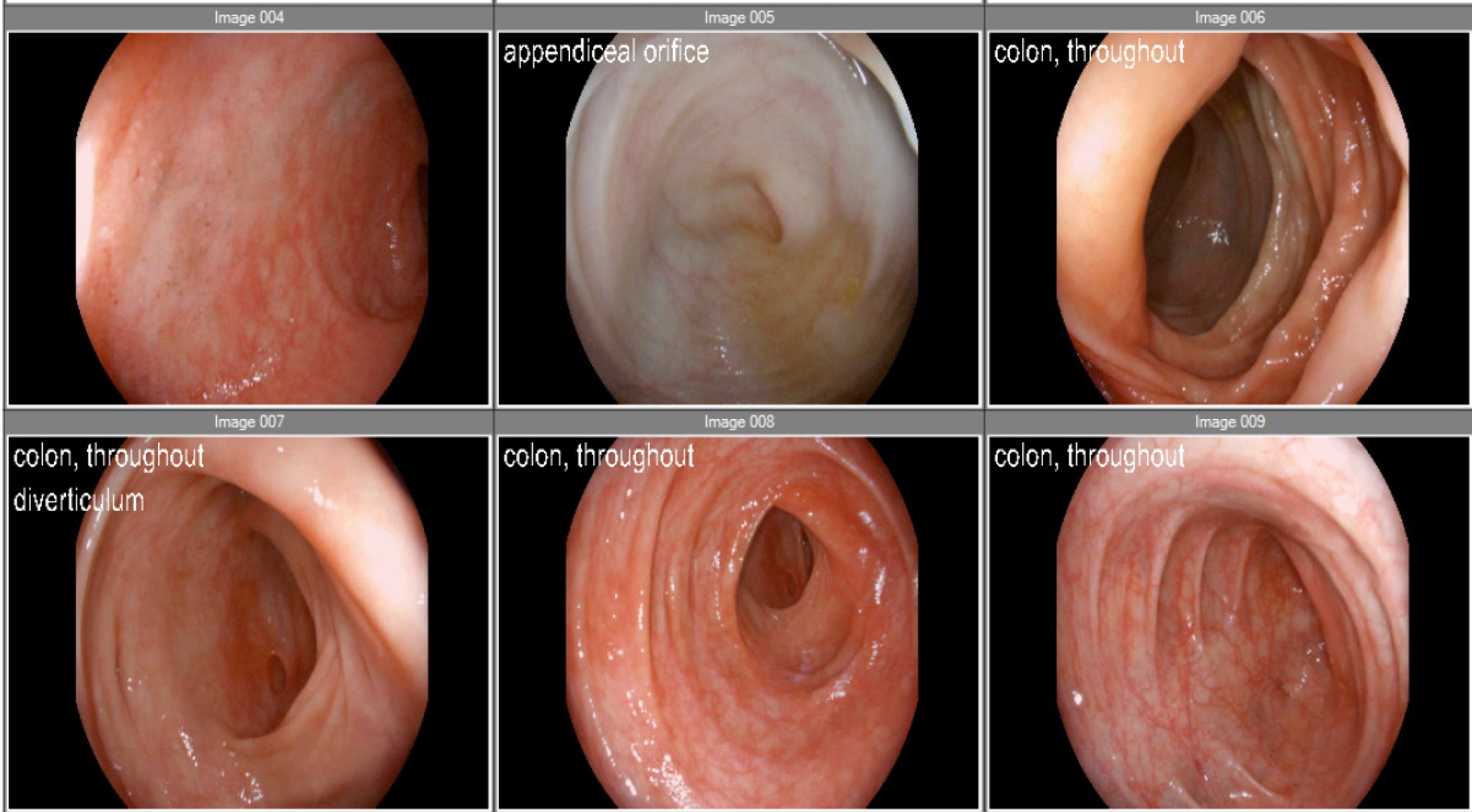
Figure: Figure 2. Colonoscopic images showing normal mucosa throughout the colon with mild diverticulosis in the sigmoid and ascending colon. No ulcerations or masses were noted. Multiple biopsies were taken despite the absence of gross abnormalities.
Disclosures:
Bara Abujaber indicated no relevant financial relationships.
Nour Abu Irhayem indicated no relevant financial relationships.
Joseph Alukal indicated no relevant financial relationships.
Mark Mattar: AbbVie – Consultant, Speakers Bureau. Celltrion – Speakers Bureau. Johnson & Johnson – Consultant, Speakers Bureau.
Bara Abujaber, MBBS1, Nour Abu Irhayem, MBBS2, Joseph Alukal, MD3, Mark Mattar, MD3. P3039 - Biopsy Beyond the Surface: A Challenging Case of Refractory Diarrhea Secondary to CAR-T Therapy, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1MedStar Georgetown/Washington Hospital Center, Washington, DC; 2MedStar Georgetown/ Washington Hospital Center, Washington, DC; 3MedStar Georgetown University Hospital, Washington, DC
Introduction: Chronic diarrhea in immunocompromised patients presents a broad range of differentials. In recipients of chimeric antigen receptor T-cell (CAR-T) therapy, delayed-onset diarrhea is often attributed to immune effector cell (IEC)–associated enterocolitis and typically responds to steroid treatment. However, persistent, steroid-refractory diarrhea may represent a rare but emerging complication: CAR-T–associated GI lymphoproliferative (LP) disorders.
Case Description/
Methods: A 70-year-old female with multiple myeloma presented with chronic, non-bloody, mucoid diarrhea ( >5 episodes/day) seven months after Cilta-cel CAR-T therapy. Her workup was negative for inflammatory, autoimmune, and common infectious causes. CMV DNA PCR was mildly elevated (555 copies/mL) and was refractory to ganciclovir and empiric antibiotics. She was later treated with steroids for suspected Graft Versus Host Disease, but diarrhea persisted. She had normal mucosa on upper and lower endoscopies, but random biopsies revealed diffuse granzyme B-positive CD8+ T-cell infiltration in the colon, duodenum, and esophagus. Sequencing verified CAR-T construct integration and clonal proliferation consistent with indolent CD8+ CAR-T cell lymphoma. She was started on IV cyclosporine 2.5 mg/kg for 14 days with symptom resolution. Although she recovered from a GI perspective, she developed influenza pneumonia and expired.
Discussion: This case highlights an extremely rare but increasingly recognized GI complication following CAR-T therapy that warrants timely diagnosis and intervention. IEC-associated enterocolitis occurs in ~1.2% of CAR-T recipients, but CAR-T–induced T-cell lymphoma following Cilta-cel has only been reported twice. The gut may act as a reservoir for clonal CAR-T expansion, with potential for systemic dissemination. Persistent diarrhea unresponsive to corticosteroids should prompt bidirectional endoscopy with biopsy. Cyclosporine is thought to treat this condition by uniquely targeting persistent engineered CAR-T cells by inhibiting IL-2-mediated mucosal proliferation and is administered at 3–5 mg/kg/day with goal troughs between 150–250 ng/mL. Given the similarity between CAR-T cell lymphoma and IBD, further research is needed on the potential role of biologics in treating this rare disease entity.
References:
- Ozdemirli et al. ICD4+ CAR T-Cell Lymphoma after CAR T Therapy. NEJM 2024
- Hamilton et al. Risk of Second Tumors and T-Cell Lymphoma after CAR T-Cell Therapy. NEJM 2024

Figure: Figure 1. Endoscopic images from esophagogastroduodenoscopy (EGD) demonstrating normal-appearing mucosa in the pylorus and duodenum despite histologic evidence of CD8+ CAR-T cell infiltration on biopsy.

Figure: Figure 2. Colonoscopic images showing normal mucosa throughout the colon with mild diverticulosis in the sigmoid and ascending colon. No ulcerations or masses were noted. Multiple biopsies were taken despite the absence of gross abnormalities.
Disclosures:
Bara Abujaber indicated no relevant financial relationships.
Nour Abu Irhayem indicated no relevant financial relationships.
Joseph Alukal indicated no relevant financial relationships.
Mark Mattar: AbbVie – Consultant, Speakers Bureau. Celltrion – Speakers Bureau. Johnson & Johnson – Consultant, Speakers Bureau.
Bara Abujaber, MBBS1, Nour Abu Irhayem, MBBS2, Joseph Alukal, MD3, Mark Mattar, MD3. P3039 - Biopsy Beyond the Surface: A Challenging Case of Refractory Diarrhea Secondary to CAR-T Therapy, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

