Monday Poster Session
Category: IBD
P3218 - The Modified Rutgeerts Score Predicts Clinical Outcomes After Primary Ileocecal Resection in Crohn’s Disease
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- RA
Rishav Agrawal, MD (he/him/his)
NewYork-Presbyterian / Weill Cornell Medical Center
New York, NY
Presenting Author(s)
Rishav Agrawal, MD1, Caitlin Mason, MS2, Jason Chua, MPH2, Michael Mintz, MD3, Laura Sahyoun, MD3, Ellen J. Scherl, MD3, Dana Lukin, MD, PhD3
1NewYork-Presbyterian / Weill Cornell Medical Center, New York, NY; 2Weill Cornell Medicine, New York, NY; 3Jill Roberts Center for Inflammatory Bowel Disease, Weill Cornell Medicine, New York, NY
Introduction: The Rutgeerts Score is used to assess recurrence in Crohn’s disease (CD) following ileocecal resection (ICR). Limited data exist relating clinical outcomes to the modified Rutgeerts Score (mRS), which differentiates isolated anastomotic lesions (i2a) from more extensive lesions of the neoterminal ileum (i2b). We aim to evaluate factors associated with index colonoscopy mRS and 12-month outcomes stratified by the mRS in CD patients after first-time ICR.
Methods: We conducted a retrospective cohort study identifying patients who underwent primary ICR and had their first post-operative colonoscopy report containing a mRS. Patients were stratified based on mRS and compared by demographics, disease characteristics, and outcomes 6-12 months post-index colonoscopy. A Harvey Bradshaw Index > 4, elevated CRP or fecal calprotectin, and imaging with active intestinal inflammation were considered symptomatic, biochemical, and imaging recurrences, respectively. At subsequent endoscopy, mRS i2b – i4 was considered endoscopic recurrence for patients with a prior mRS of i0 – i2a or persistent endoscopic activity for patients with a prior mRS of i2b – i4. Chi Square and Fisher Exact tests were used to compare categorical variables of interest and associations of continuous variables were examined via T-tests or Mann-Whitney U tests.
Results: 138 patients were identified meeting inclusion criteria, 96 with a mRS of i0 – i2a and 42 with a mRS of i2b – i4. Patients with a mRS of i2b – i4 were more likely to be above the age of 40 at time of CD diagnosis, had an older age at surgery, were more likely to have an end-to-side anastomosis, and had a longer interval between ICR and their first post-operative colonoscopy compared mRS i0 – i2a patients (Table 1). Among those with relevant follow up, i2b – i4 patients had higher rates of symptomatic (p < 0.01) and biochemical recurrence (p < 0.01) at 6-12 months after index colonoscopy, and there were numerically higher rates of imaging and endoscopic activity (p= NS; Table 2).
Discussion: The mRS is strongly associated with outcomes in CD after ICR. Patients with an older age at surgery or over 40 years of age at CD diagnosis may benefit from closer post-operative follow up, and end-to-side anastomoses may carry a higher risk of recurrence. After index colonoscopy, patients with a mRS i2b-i4 should have more proactive surveillance for disease activity. Further prospective studies are needed to validate these observations.
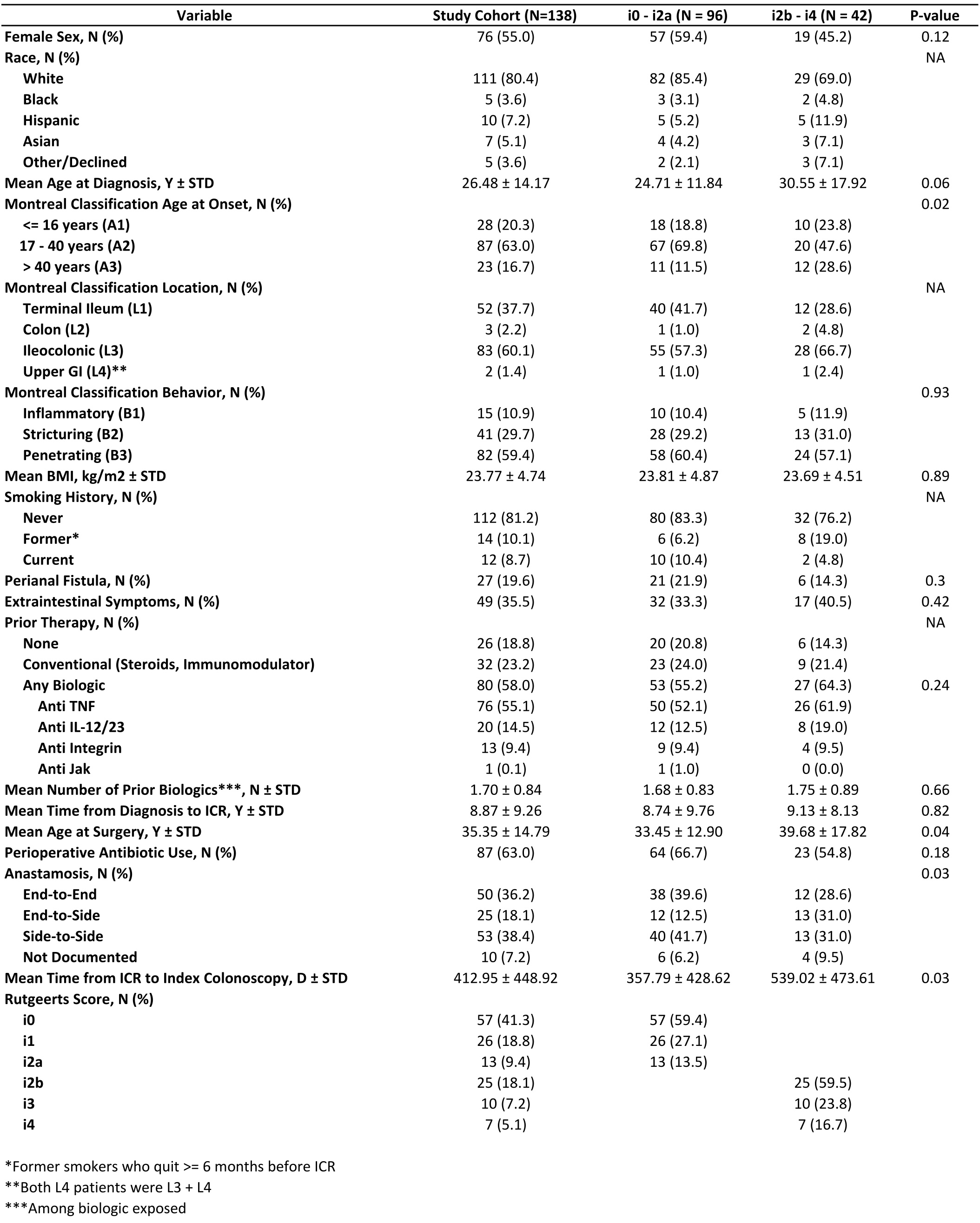
Figure: Table 1. Baseline characteristics
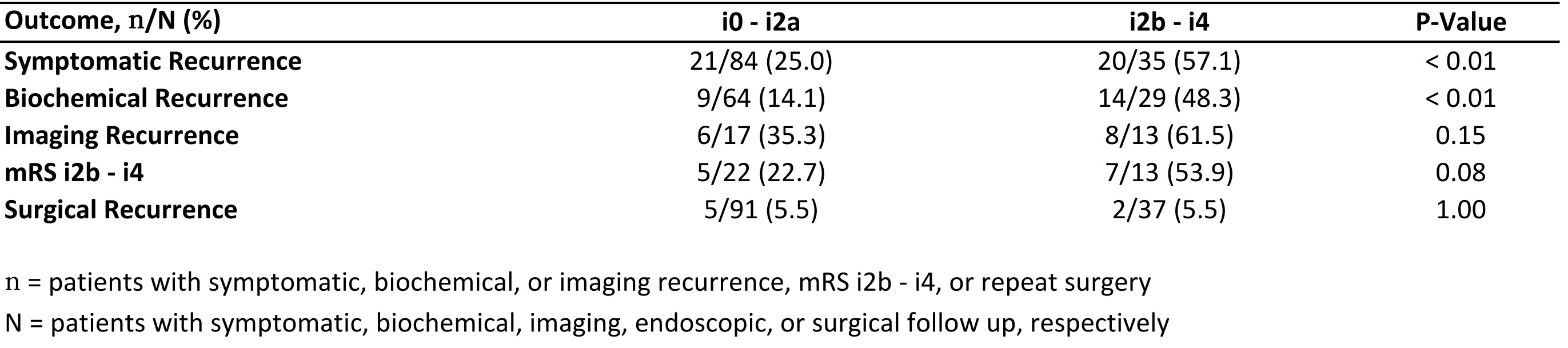
Figure: Table 2. One year outcomes by modified Rutgeerts score
Disclosures:
Rishav Agrawal indicated no relevant financial relationships.
Caitlin Mason indicated no relevant financial relationships.
Jason Chua indicated no relevant financial relationships.
Michael Mintz indicated no relevant financial relationships.
Laura Sahyoun indicated no relevant financial relationships.
Ellen Scherl indicated no relevant financial relationships.
Dana Lukin: Abbvie – Consultant, Grant/Research Support, Speakers Bureau. Altrubio – Consultant. Boehringer Ingelheim – Consultant, Grant/Research Support. Bristol Myers Squibb – Consultant. Johnson & Johnson – Consultant, Grant/Research Support, Speakers Bureau. Palatin Technologies – Consultant. Pfizer – Consultant. Prime – Consultant. PSI – Consultant. Takeda – Consultant. Vedanta – Consultant.
Rishav Agrawal, MD1, Caitlin Mason, MS2, Jason Chua, MPH2, Michael Mintz, MD3, Laura Sahyoun, MD3, Ellen J. Scherl, MD3, Dana Lukin, MD, PhD3. P3218 - The Modified Rutgeerts Score Predicts Clinical Outcomes After Primary Ileocecal Resection in Crohn’s Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1NewYork-Presbyterian / Weill Cornell Medical Center, New York, NY; 2Weill Cornell Medicine, New York, NY; 3Jill Roberts Center for Inflammatory Bowel Disease, Weill Cornell Medicine, New York, NY
Introduction: The Rutgeerts Score is used to assess recurrence in Crohn’s disease (CD) following ileocecal resection (ICR). Limited data exist relating clinical outcomes to the modified Rutgeerts Score (mRS), which differentiates isolated anastomotic lesions (i2a) from more extensive lesions of the neoterminal ileum (i2b). We aim to evaluate factors associated with index colonoscopy mRS and 12-month outcomes stratified by the mRS in CD patients after first-time ICR.
Methods: We conducted a retrospective cohort study identifying patients who underwent primary ICR and had their first post-operative colonoscopy report containing a mRS. Patients were stratified based on mRS and compared by demographics, disease characteristics, and outcomes 6-12 months post-index colonoscopy. A Harvey Bradshaw Index > 4, elevated CRP or fecal calprotectin, and imaging with active intestinal inflammation were considered symptomatic, biochemical, and imaging recurrences, respectively. At subsequent endoscopy, mRS i2b – i4 was considered endoscopic recurrence for patients with a prior mRS of i0 – i2a or persistent endoscopic activity for patients with a prior mRS of i2b – i4. Chi Square and Fisher Exact tests were used to compare categorical variables of interest and associations of continuous variables were examined via T-tests or Mann-Whitney U tests.
Results: 138 patients were identified meeting inclusion criteria, 96 with a mRS of i0 – i2a and 42 with a mRS of i2b – i4. Patients with a mRS of i2b – i4 were more likely to be above the age of 40 at time of CD diagnosis, had an older age at surgery, were more likely to have an end-to-side anastomosis, and had a longer interval between ICR and their first post-operative colonoscopy compared mRS i0 – i2a patients (Table 1). Among those with relevant follow up, i2b – i4 patients had higher rates of symptomatic (p < 0.01) and biochemical recurrence (p < 0.01) at 6-12 months after index colonoscopy, and there were numerically higher rates of imaging and endoscopic activity (p= NS; Table 2).
Discussion: The mRS is strongly associated with outcomes in CD after ICR. Patients with an older age at surgery or over 40 years of age at CD diagnosis may benefit from closer post-operative follow up, and end-to-side anastomoses may carry a higher risk of recurrence. After index colonoscopy, patients with a mRS i2b-i4 should have more proactive surveillance for disease activity. Further prospective studies are needed to validate these observations.

Figure: Table 1. Baseline characteristics

Figure: Table 2. One year outcomes by modified Rutgeerts score
Disclosures:
Rishav Agrawal indicated no relevant financial relationships.
Caitlin Mason indicated no relevant financial relationships.
Jason Chua indicated no relevant financial relationships.
Michael Mintz indicated no relevant financial relationships.
Laura Sahyoun indicated no relevant financial relationships.
Ellen Scherl indicated no relevant financial relationships.
Dana Lukin: Abbvie – Consultant, Grant/Research Support, Speakers Bureau. Altrubio – Consultant. Boehringer Ingelheim – Consultant, Grant/Research Support. Bristol Myers Squibb – Consultant. Johnson & Johnson – Consultant, Grant/Research Support, Speakers Bureau. Palatin Technologies – Consultant. Pfizer – Consultant. Prime – Consultant. PSI – Consultant. Takeda – Consultant. Vedanta – Consultant.
Rishav Agrawal, MD1, Caitlin Mason, MS2, Jason Chua, MPH2, Michael Mintz, MD3, Laura Sahyoun, MD3, Ellen J. Scherl, MD3, Dana Lukin, MD, PhD3. P3218 - The Modified Rutgeerts Score Predicts Clinical Outcomes After Primary Ileocecal Resection in Crohn’s Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

