Monday Poster Session
Category: IBD
P3201 - Autologous Hematopoietic Stem Cell Transplantation (autoHSCT) for Crohn’s Disease
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Jana G. Al Hashash, MD, MSc, FACG
Associate Professor
Mayo Clinic
Jacksonville, FL
Presenting Author(s)
Jana G. Hashash, MD, MSc, FACG1, Madiha Iqbal, MD1, Mohamed Kharfan-Dabaja, MD1, Jami Kinnucan, MD, FACG1, Konstantinos Papadakis, MD2, Vivek Roy, MD1, James Foran, MD1, Hermant Murthy, MD1, Ricardo Parrondo, MD1, Francis A.. Farraye, MD, MSc, MACG1, William Faubion, MD1, Michael Picco, MD, PhD1, Ernesto Ayala, MD1
1Mayo Clinic, Jacksonville, FL; 2Mayo Clinic, Rochester, MN
Introduction: Despite available therapies, a subset of patients with Crohn’s disease (CD) have a refractory course. The profound immune suppresion from preparative regimens prior to autologous hematopoietic cell transplantation (autoHCT) induces depletion of self-reactive T- and B-cells, consequently attenuating the inflammatory process and resetting the dysbalanced immune response. AutoHCT uses a patient’s own hematopoietic stem cells and has been used to treat CD. We present interim results of our ongoing study using autoHCT for treating medically refractory CD.
Methods: This is a prospective observational case series of adult patients with medically refractory CD who underwent autoHCT at our institution. Clinical, laboratory, radiographic, and endoscopic data were collected before, during, and at different intervals post-autoHCT. Peripheral blood hematopoietic stem cells were collected after mobilization with filgrastim±plerixafor. Then, patients were admitted to the BMT unit. Conditioning regimen included cyclophosphamide 50 mg/kg/day (days -5 to -2) and rabbit anti-thymocyte globulin 0.5 mg/kg on day -5, followed by 1.5 mg/kg/day (days -4 to -1). Autologous stem cells were CD34+ selected in all patients and infused on day 0. Infection prophylaxis and treatment followed established institutional guidelines. All patients underwent a preemptive surveillance approach to identify CMV and EBV reactivation weekly until day +60. CD was reassessed at day +90, with noninvasive biomarkers, imaging and/or endoscopy.
Results: Five patients underwent autoHCT with no life threatening adverse events. CD healing response rates as measured by biochemical, clinical, radiographic, and endoscopic, among these patients varied. Patient demographics and CD-related clinical history are shown in Table 1. Outcomes of the patients are shown in Table 2.
Discussion: In our series, autoHCT was safe and associated with relatively low morbidity. Side effects included volume overload during hydration for cyclophosphamide, 1 episode of bacteremia, and 1 readmission for persistent nausea/vomiting. No flares of CD occurred during the transplant process. No EBV or CMV reactivations occurred. Regarding CD, there was improvement of symptoms, reduction of inflammatory markers and improvement in quality of life. All patients recovered from the autoHCT and 1 patient remained free of CD directed therapy for 12 months post transplant. High dose immune ablation with autologous transplantation remains a promising therapy for refractory CD.
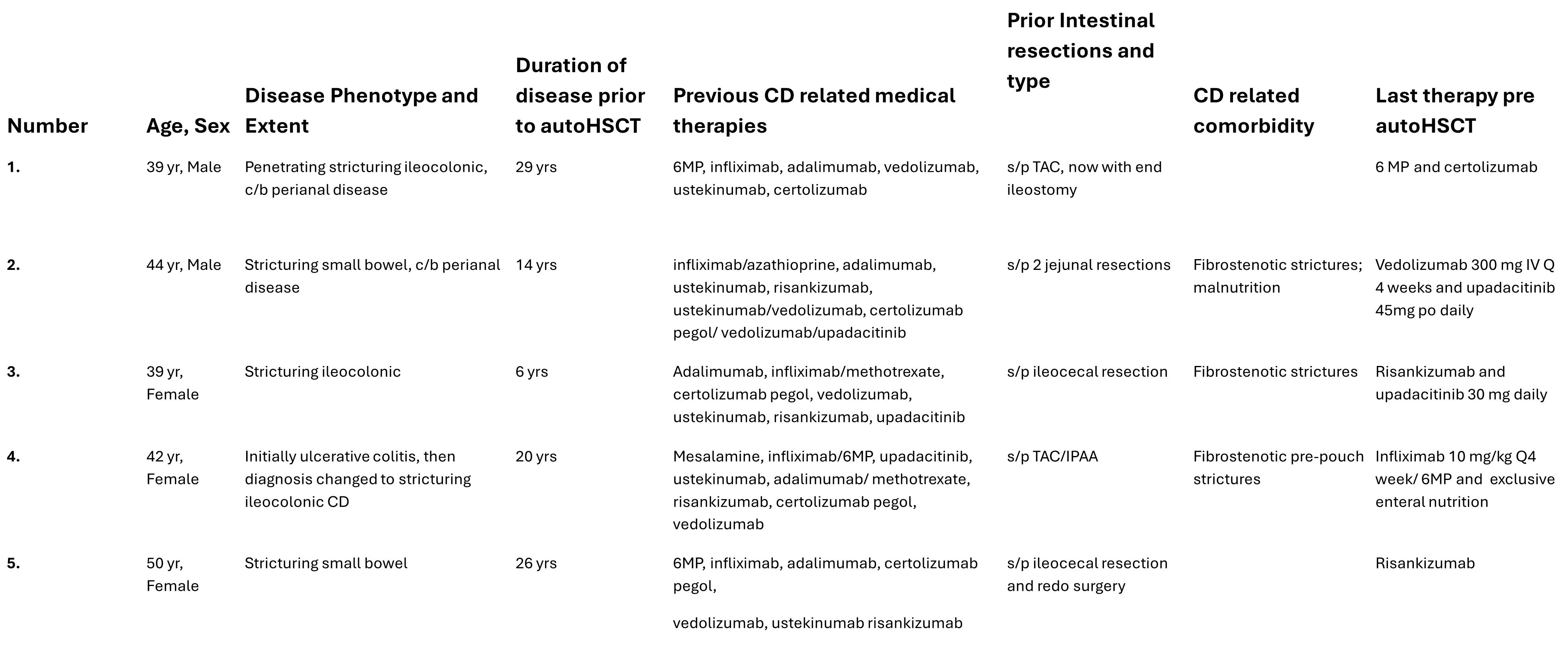
Figure: Table 1. Patient Demographics and CD Related History
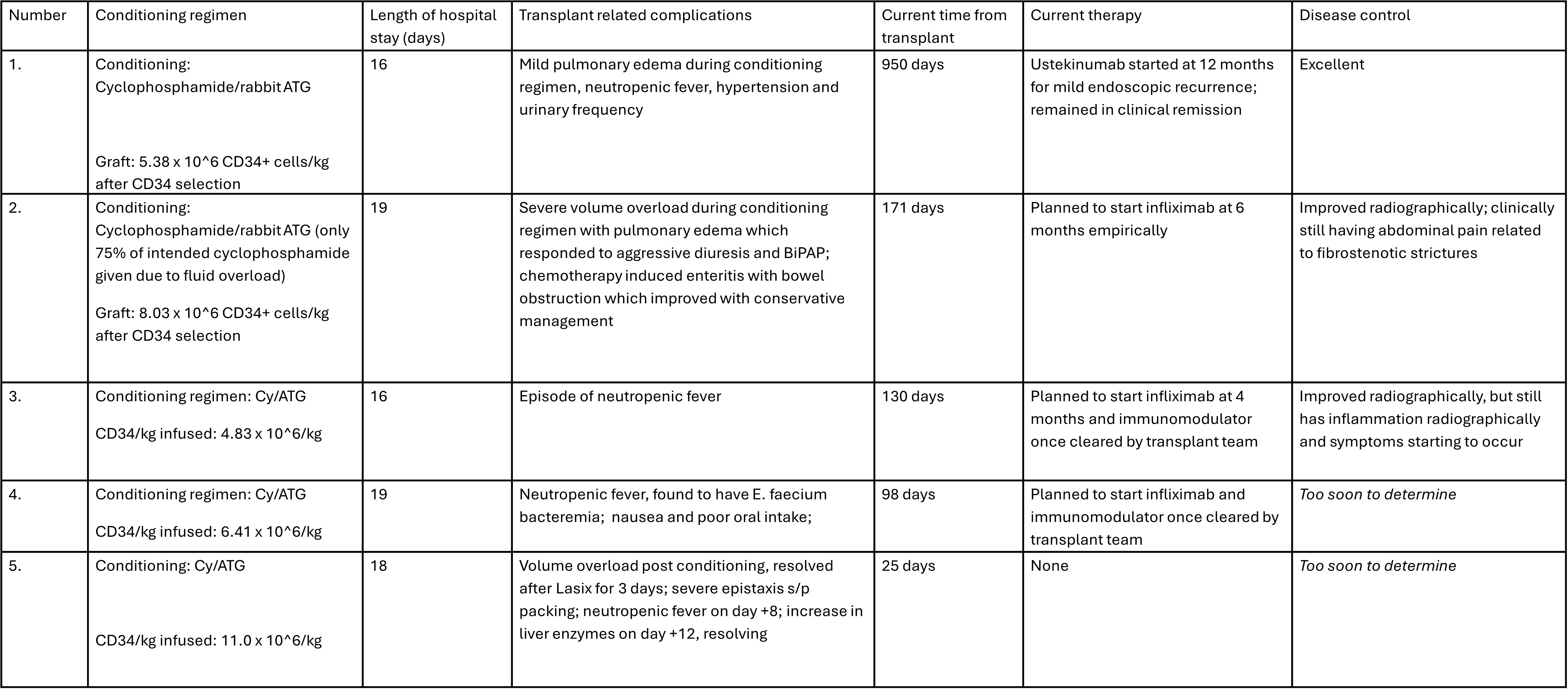
Figure: Table 2. Transplant Details and Outcomes
Disclosures:
Jana Hashash: BMS – Ad Board.
Madiha Iqbal indicated no relevant financial relationships.
Mohamed Kharfan-Dabaja indicated no relevant financial relationships.
Jami Kinnucan: Abbvie – Advisor or Review Panel Member, Consultant. J&J – Advisor or Review Panel Member. Lilly – Advisor or Review Panel Member, Consultant. Pfizer – Advisor or Review Panel Member, Consultant. Takeda – Advisor or Review Panel Member, Consultant.
Konstantinos Papadakis: Amgen – Stock-publicly held company(excluding mutual/index funds). JNJ – Stock-publicly held company(excluding mutual/index funds). Merck – Stock-publicly held company(excluding mutual/index funds).
Vivek Roy indicated no relevant financial relationships.
James Foran indicated no relevant financial relationships.
Hermant Murthy indicated no relevant financial relationships.
Ricardo Parrondo indicated no relevant financial relationships.
Francis Farraye: Astellas – Advisory Committee/Board Member. Avalo – Advisory Committee/Board Member. Bausch – Advisory Committee/Board Member. BMS – Advisory Committee/Board Member. Braintree Labs – Advisory Committee/Board Member. Fresenius Kabi – Advisory Committee/Board Member. GI Reviewers – Independent Contractor. IBD Educational Group – Independent Contractor. Iterative Health – Advisory Committee/Board Member, Stock Options. Janssen – Advisory Committee/Board Member. Lilly – DSMB. Pfizer – Advisory Committee/Board Member. Pharmacosmos – Advisory Committee/Board Member. Sandoz – Advisory Committee/Board Member. Viatris – Advisory Committee/Board Member.
William Faubion indicated no relevant financial relationships.
Michael Picco indicated no relevant financial relationships.
Ernesto Ayala indicated no relevant financial relationships.
Jana G. Hashash, MD, MSc, FACG1, Madiha Iqbal, MD1, Mohamed Kharfan-Dabaja, MD1, Jami Kinnucan, MD, FACG1, Konstantinos Papadakis, MD2, Vivek Roy, MD1, James Foran, MD1, Hermant Murthy, MD1, Ricardo Parrondo, MD1, Francis A.. Farraye, MD, MSc, MACG1, William Faubion, MD1, Michael Picco, MD, PhD1, Ernesto Ayala, MD1. P3201 - Autologous Hematopoietic Stem Cell Transplantation (autoHSCT) for Crohn’s Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Mayo Clinic, Jacksonville, FL; 2Mayo Clinic, Rochester, MN
Introduction: Despite available therapies, a subset of patients with Crohn’s disease (CD) have a refractory course. The profound immune suppresion from preparative regimens prior to autologous hematopoietic cell transplantation (autoHCT) induces depletion of self-reactive T- and B-cells, consequently attenuating the inflammatory process and resetting the dysbalanced immune response. AutoHCT uses a patient’s own hematopoietic stem cells and has been used to treat CD. We present interim results of our ongoing study using autoHCT for treating medically refractory CD.
Methods: This is a prospective observational case series of adult patients with medically refractory CD who underwent autoHCT at our institution. Clinical, laboratory, radiographic, and endoscopic data were collected before, during, and at different intervals post-autoHCT. Peripheral blood hematopoietic stem cells were collected after mobilization with filgrastim±plerixafor. Then, patients were admitted to the BMT unit. Conditioning regimen included cyclophosphamide 50 mg/kg/day (days -5 to -2) and rabbit anti-thymocyte globulin 0.5 mg/kg on day -5, followed by 1.5 mg/kg/day (days -4 to -1). Autologous stem cells were CD34+ selected in all patients and infused on day 0. Infection prophylaxis and treatment followed established institutional guidelines. All patients underwent a preemptive surveillance approach to identify CMV and EBV reactivation weekly until day +60. CD was reassessed at day +90, with noninvasive biomarkers, imaging and/or endoscopy.
Results: Five patients underwent autoHCT with no life threatening adverse events. CD healing response rates as measured by biochemical, clinical, radiographic, and endoscopic, among these patients varied. Patient demographics and CD-related clinical history are shown in Table 1. Outcomes of the patients are shown in Table 2.
Discussion: In our series, autoHCT was safe and associated with relatively low morbidity. Side effects included volume overload during hydration for cyclophosphamide, 1 episode of bacteremia, and 1 readmission for persistent nausea/vomiting. No flares of CD occurred during the transplant process. No EBV or CMV reactivations occurred. Regarding CD, there was improvement of symptoms, reduction of inflammatory markers and improvement in quality of life. All patients recovered from the autoHCT and 1 patient remained free of CD directed therapy for 12 months post transplant. High dose immune ablation with autologous transplantation remains a promising therapy for refractory CD.

Figure: Table 1. Patient Demographics and CD Related History

Figure: Table 2. Transplant Details and Outcomes
Disclosures:
Jana Hashash: BMS – Ad Board.
Madiha Iqbal indicated no relevant financial relationships.
Mohamed Kharfan-Dabaja indicated no relevant financial relationships.
Jami Kinnucan: Abbvie – Advisor or Review Panel Member, Consultant. J&J – Advisor or Review Panel Member. Lilly – Advisor or Review Panel Member, Consultant. Pfizer – Advisor or Review Panel Member, Consultant. Takeda – Advisor or Review Panel Member, Consultant.
Konstantinos Papadakis: Amgen – Stock-publicly held company(excluding mutual/index funds). JNJ – Stock-publicly held company(excluding mutual/index funds). Merck – Stock-publicly held company(excluding mutual/index funds).
Vivek Roy indicated no relevant financial relationships.
James Foran indicated no relevant financial relationships.
Hermant Murthy indicated no relevant financial relationships.
Ricardo Parrondo indicated no relevant financial relationships.
Francis Farraye: Astellas – Advisory Committee/Board Member. Avalo – Advisory Committee/Board Member. Bausch – Advisory Committee/Board Member. BMS – Advisory Committee/Board Member. Braintree Labs – Advisory Committee/Board Member. Fresenius Kabi – Advisory Committee/Board Member. GI Reviewers – Independent Contractor. IBD Educational Group – Independent Contractor. Iterative Health – Advisory Committee/Board Member, Stock Options. Janssen – Advisory Committee/Board Member. Lilly – DSMB. Pfizer – Advisory Committee/Board Member. Pharmacosmos – Advisory Committee/Board Member. Sandoz – Advisory Committee/Board Member. Viatris – Advisory Committee/Board Member.
William Faubion indicated no relevant financial relationships.
Michael Picco indicated no relevant financial relationships.
Ernesto Ayala indicated no relevant financial relationships.
Jana G. Hashash, MD, MSc, FACG1, Madiha Iqbal, MD1, Mohamed Kharfan-Dabaja, MD1, Jami Kinnucan, MD, FACG1, Konstantinos Papadakis, MD2, Vivek Roy, MD1, James Foran, MD1, Hermant Murthy, MD1, Ricardo Parrondo, MD1, Francis A.. Farraye, MD, MSc, MACG1, William Faubion, MD1, Michael Picco, MD, PhD1, Ernesto Ayala, MD1. P3201 - Autologous Hematopoietic Stem Cell Transplantation (autoHSCT) for Crohn’s Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
