Monday Poster Session
Category: IBD
P3287 - EXE-346 Live Biotherapeutic Reduces High Bowel Movement Frequency in Subjects With an Ileal Pouch-Anal Anastomosis
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Edward L. Barnes, MD, MPH
Multidisciplinary Inflammatory Bowel Diseases Center, Division of Gastroenterology and Hepatology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
Chapel Hill, NC
Presenting Author(s)
Award: ACG Presidential Poster Award
Hans Herfarth, MD, PhD1, Shannon Chang, MD2, Darrell Pardi, MD, MS, FACG3, Edward Barnes, MD, MPH1
1University of North Carolina, Chapel Hill, NC; 2New York University Langone Health, New York, NY, USA, New York, NY; 3Mayo Clinic, Rochester, MN
Introduction: EXE-346 is an optimized, high-dose, biologic grade formulation of the commercially available food-grade probiotic VisbiomeÒ. Currently, there are limited medical options to manage increased bowel frequency in patients with ileal pouch-anal anastomosis (IPAA), which often occurs independent of inflammation. To address this unmet need, we conducted an open-label, single-arm, multi-center Phase 1B study involving patients with refractory high bowel frequency. The objective of the study was to evaluate the safety and preliminary efficacy of EXE-346 in this patient population.
Methods: IPAA patients with chronically elevated bowel frequency and a 24-hour average bowel frequency (ABF) of ≥10 during a 7-day screening period, were eligible to enter the study. The patients were treated with EXE-346 (1500×10⁹ CFU) twice daily for four weeks. Bowel frequency was recorded daily throughout the study. Main study exclusion criteria were a diagnosis of Crohn’s-like disease of the pouch, known treatment-refractory pouch-anal strictures, concomitant therapy with biologics or systemic corticosteroids, or a positive Clostridoides difficile assay. Data are reported as median and interquartile range (IQR). Statistical analyses were performed using the Wilcoxon signed-rank test between baseline measurements and subsequent time point.
Results: At 4 US centers, 10 patients with a median baseline ABF of 11.7 (IQR 10.5-13.6) completed the 4-week study. EXE-346 was overall well tolerated; side effects occurred in 4 of the 10 patients (n=1 for each: decreased appetite, rash, elevation of serum calcium, and worsening of liver function tests in a patient with hepatic steatosis), all of which were judged mild in severity. Compared to baseline, the median ABF decreased by 23% to 9.1 (IQR 7.9-12.1) at week 4 (p=0.002; Figure 1). The nighttime bowel frequency decreased by 48% from a median of 2.3 (IQR 1.3-3.5) at baseline to 1.2 (IQR 0.9-2.7) at week 4 (p< 0.01).
Discussion: EXE-346 was safe and well tolerated, with only mild adverse events reported. Treatment with EXE-346 resulted in a clinically meaningful reduction in overall average bowel frequency and nighttime bowel frequency. These preliminary findings support the potential of EXE-346 to improve symptoms in patients with elevated ABF and warrant further evaluation in a placebo-controlled Phase 2 study, which is currently underway.
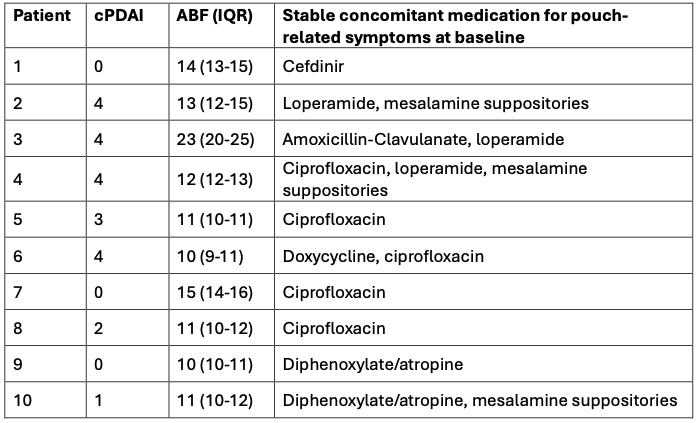
Figure: Figure 1
Box plots illustrating the distribution of bowel movement frequency among ten patients over four weeks of EXE-346 treatment.
Each box plot provides a visual representation of the data, with horizontal lines indicating the minimum, first quartile, median, third quartile, and maximum values. Additionally, the results of the Wilcoxon signed-rank test, conducted between baseline measurements and subsequent time points, indicate statistically significant differences at alpha level of 0.05.
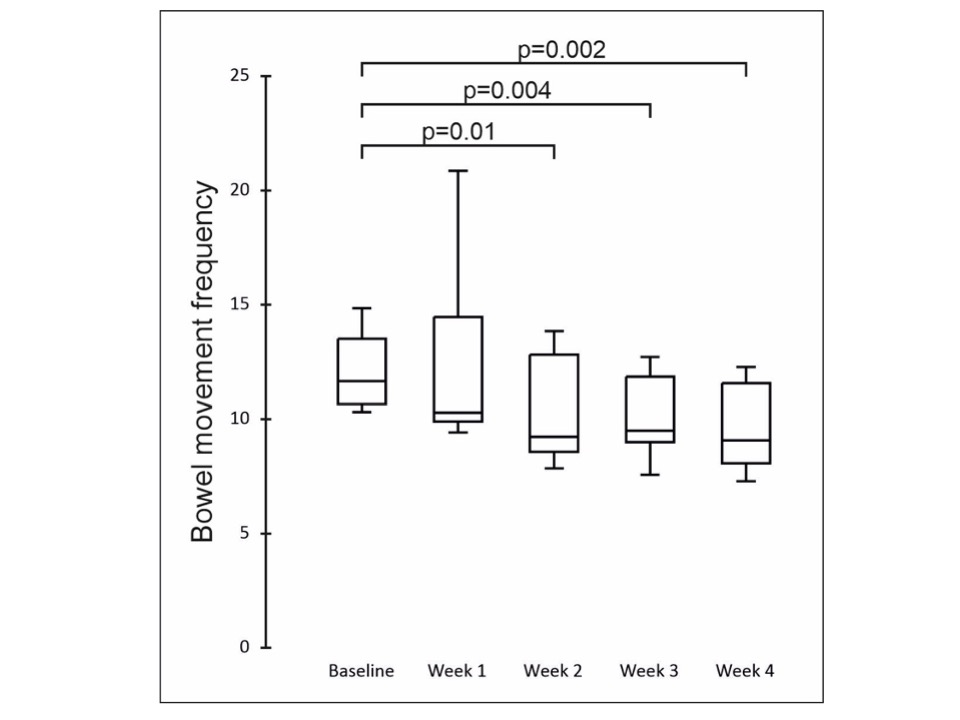
Figure: Table 1: Baseline 24-hour average bowel frequency (ABF), clinical pouch disease activity index (cPDAI) and concomitant medications for pouch-related symptoms in the study population.
Disclosures:
Hans Herfarth: Celltrion – Consultant. ExeGi – Consultant. Gilead – Consultant. Lilly – Grant/Research Support. Novo Nordisk – Grant/Research Support.
Shannon Chang: AbbVie – Consultant. BMS – Ad board. Janssen – Ad board. Lilly – Ad board. Pfizer – Ad board.
Darrell Pardi: Applied Molecular Transport – Consultant. ExeGI Pharma LC – Grant/Research Support. Janssen – Advisory Committee/Board Member. Lilly Medical – Consultant. Pfizer – Consultant. Rise Therapeutics – Grant/Research Support. Takeda – Consultant. Vedanta Bio Sciences INC – Grant/Research Support.
Edward Barnes: AbbVie, Inc. – Consultant. Boomerang – Consultant. Johnson & Johnson – Consultant. Pfizer – Consultant. Sanofi – Consultant. Takeda – Advisory Committee/Board Member. Target RWE – Consultant.
Hans Herfarth, MD, PhD1, Shannon Chang, MD2, Darrell Pardi, MD, MS, FACG3, Edward Barnes, MD, MPH1. P3287 - EXE-346 Live Biotherapeutic Reduces High Bowel Movement Frequency in Subjects With an Ileal Pouch-Anal Anastomosis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Hans Herfarth, MD, PhD1, Shannon Chang, MD2, Darrell Pardi, MD, MS, FACG3, Edward Barnes, MD, MPH1
1University of North Carolina, Chapel Hill, NC; 2New York University Langone Health, New York, NY, USA, New York, NY; 3Mayo Clinic, Rochester, MN
Introduction: EXE-346 is an optimized, high-dose, biologic grade formulation of the commercially available food-grade probiotic VisbiomeÒ. Currently, there are limited medical options to manage increased bowel frequency in patients with ileal pouch-anal anastomosis (IPAA), which often occurs independent of inflammation. To address this unmet need, we conducted an open-label, single-arm, multi-center Phase 1B study involving patients with refractory high bowel frequency. The objective of the study was to evaluate the safety and preliminary efficacy of EXE-346 in this patient population.
Methods: IPAA patients with chronically elevated bowel frequency and a 24-hour average bowel frequency (ABF) of ≥10 during a 7-day screening period, were eligible to enter the study. The patients were treated with EXE-346 (1500×10⁹ CFU) twice daily for four weeks. Bowel frequency was recorded daily throughout the study. Main study exclusion criteria were a diagnosis of Crohn’s-like disease of the pouch, known treatment-refractory pouch-anal strictures, concomitant therapy with biologics or systemic corticosteroids, or a positive Clostridoides difficile assay. Data are reported as median and interquartile range (IQR). Statistical analyses were performed using the Wilcoxon signed-rank test between baseline measurements and subsequent time point.
Results: At 4 US centers, 10 patients with a median baseline ABF of 11.7 (IQR 10.5-13.6) completed the 4-week study. EXE-346 was overall well tolerated; side effects occurred in 4 of the 10 patients (n=1 for each: decreased appetite, rash, elevation of serum calcium, and worsening of liver function tests in a patient with hepatic steatosis), all of which were judged mild in severity. Compared to baseline, the median ABF decreased by 23% to 9.1 (IQR 7.9-12.1) at week 4 (p=0.002; Figure 1). The nighttime bowel frequency decreased by 48% from a median of 2.3 (IQR 1.3-3.5) at baseline to 1.2 (IQR 0.9-2.7) at week 4 (p< 0.01).
Discussion: EXE-346 was safe and well tolerated, with only mild adverse events reported. Treatment with EXE-346 resulted in a clinically meaningful reduction in overall average bowel frequency and nighttime bowel frequency. These preliminary findings support the potential of EXE-346 to improve symptoms in patients with elevated ABF and warrant further evaluation in a placebo-controlled Phase 2 study, which is currently underway.

Figure: Figure 1
Box plots illustrating the distribution of bowel movement frequency among ten patients over four weeks of EXE-346 treatment.
Each box plot provides a visual representation of the data, with horizontal lines indicating the minimum, first quartile, median, third quartile, and maximum values. Additionally, the results of the Wilcoxon signed-rank test, conducted between baseline measurements and subsequent time points, indicate statistically significant differences at alpha level of 0.05.

Figure: Table 1: Baseline 24-hour average bowel frequency (ABF), clinical pouch disease activity index (cPDAI) and concomitant medications for pouch-related symptoms in the study population.
Disclosures:
Hans Herfarth: Celltrion – Consultant. ExeGi – Consultant. Gilead – Consultant. Lilly – Grant/Research Support. Novo Nordisk – Grant/Research Support.
Shannon Chang: AbbVie – Consultant. BMS – Ad board. Janssen – Ad board. Lilly – Ad board. Pfizer – Ad board.
Darrell Pardi: Applied Molecular Transport – Consultant. ExeGI Pharma LC – Grant/Research Support. Janssen – Advisory Committee/Board Member. Lilly Medical – Consultant. Pfizer – Consultant. Rise Therapeutics – Grant/Research Support. Takeda – Consultant. Vedanta Bio Sciences INC – Grant/Research Support.
Edward Barnes: AbbVie, Inc. – Consultant. Boomerang – Consultant. Johnson & Johnson – Consultant. Pfizer – Consultant. Sanofi – Consultant. Takeda – Advisory Committee/Board Member. Target RWE – Consultant.
Hans Herfarth, MD, PhD1, Shannon Chang, MD2, Darrell Pardi, MD, MS, FACG3, Edward Barnes, MD, MPH1. P3287 - EXE-346 Live Biotherapeutic Reduces High Bowel Movement Frequency in Subjects With an Ileal Pouch-Anal Anastomosis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

