Monday Poster Session
Category: IBD
P3284 - The Utility of Routine Laboratory Surveillance for Patients With Inflammatory Bowel Disease on Biologic or Small Molecule Therapy and Associated Costs
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Adam M. Burton, MD
Brown University / Rhode Island Hospital
Providence, RI
Presenting Author(s)
Adam M. Burton, MD1, Nicholas Scalzo, MD1, Christina Raker, MSc(Epi)2, Alex Chang, MD1, Kimberly Ho, MD1, Aaron Seto, BS3, Cara Sammartino, PhD, MSPH2, Sean Fine, MD1
1Brown University / Rhode Island Hospital, Providence, RI; 2Lifespan Health System, Providence, RI; 3Brown University, Providence, RI
Introduction: Biologic and small molecule drugs have become the mainstay of current treatment in IBD. Due to potential concern for adverse reactions associated with these medications, routine lab monitoring is recommended in the medication labeling. While cardio-thrombotic, hematologic and hepatic side effects exist, there remains insufficient data guiding how frequently clinicians should obtain routine lab monitoring for patients stable on these medications. Our aim was to describe the real-world incidence of serologic abnormalities arising from routine lab surveillance in IBD patients stable on biologic or small molecule therapy.
Methods: We did retrospective analysis from a single adult IBD academic center from 2021 to 2025. Inclusion criteria consisted of clinical stability, which was defined as patients on biologic or small molecule therapy for at least 6 months or more without hospitalization, need for steroids, or change in dosage/frequency of medication. Patients with Crohn’s Disease (CD) and Ulcerative Colitis (UC) were included in the study. We reviewed routine labs ordered by the IBD clinic including CBC, LFT, Lipid panel, and Iron panel. Abnormalities were defined as being outside of the normal reference range per Brown Health Laboratory guidelines. Data was securely stored using REDCap as part of an IRB-approved study. Descriptive statistics were performed using STATA MP18. Lab costs were estimated using the average of two major labs in RI and follow-up visits were based on average physician visit cost in RI.
Results: A total of 124 patients were analyzed in the study (Table 1). Over the study period, 817 labs were drawn ($45,279 total cost, $228 median per patient), 250 of which detected abnormalities (30.6%). However, only 15 (6%) of these abnormalities led to further workup (Table 2). One patient developed warm autoimmune hemolytic anemia, suspected related to Golimumab. However, the remainder of the lab abnormalities prompting further workup, costing another $55,310, were unrelated to their IBD medication.
Discussion: Our data suggests that laboratory surveillance monitoring may find abnormalities in about 1/4 tests, but these abnormalities may not be clinically related to their IBD medication and may be contributing to unnecessary healthcare cost. Further study is needed to guide clinicians on how frequently to monitor standard labs for IBD patients stable on their maintenance biologic or small molecule therapy.
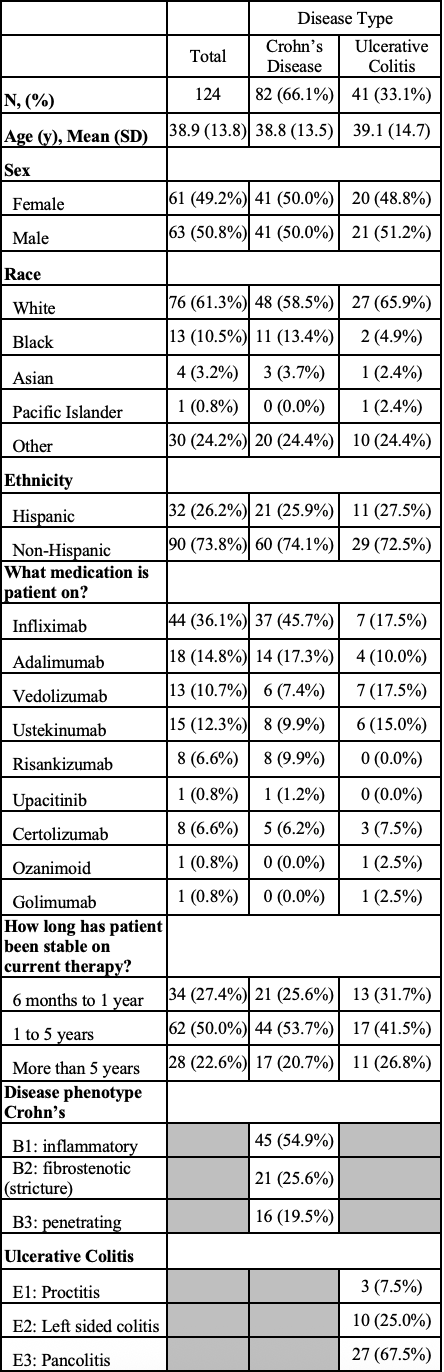
Figure: Table 1: Study baseline patient characteristics

Figure: Abnormalities detected on routine lab monitoring and those that prompted further workup.
Disclosures:
Adam Burton indicated no relevant financial relationships.
Nicholas Scalzo indicated no relevant financial relationships.
Christina Raker indicated no relevant financial relationships.
Alex Chang indicated no relevant financial relationships.
Kimberly Ho indicated no relevant financial relationships.
Aaron Seto indicated no relevant financial relationships.
Cara Sammartino indicated no relevant financial relationships.
Sean Fine indicated no relevant financial relationships.
Adam M. Burton, MD1, Nicholas Scalzo, MD1, Christina Raker, MSc(Epi)2, Alex Chang, MD1, Kimberly Ho, MD1, Aaron Seto, BS3, Cara Sammartino, PhD, MSPH2, Sean Fine, MD1. P3284 - The Utility of Routine Laboratory Surveillance for Patients With Inflammatory Bowel Disease on Biologic or Small Molecule Therapy and Associated Costs, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Brown University / Rhode Island Hospital, Providence, RI; 2Lifespan Health System, Providence, RI; 3Brown University, Providence, RI
Introduction: Biologic and small molecule drugs have become the mainstay of current treatment in IBD. Due to potential concern for adverse reactions associated with these medications, routine lab monitoring is recommended in the medication labeling. While cardio-thrombotic, hematologic and hepatic side effects exist, there remains insufficient data guiding how frequently clinicians should obtain routine lab monitoring for patients stable on these medications. Our aim was to describe the real-world incidence of serologic abnormalities arising from routine lab surveillance in IBD patients stable on biologic or small molecule therapy.
Methods: We did retrospective analysis from a single adult IBD academic center from 2021 to 2025. Inclusion criteria consisted of clinical stability, which was defined as patients on biologic or small molecule therapy for at least 6 months or more without hospitalization, need for steroids, or change in dosage/frequency of medication. Patients with Crohn’s Disease (CD) and Ulcerative Colitis (UC) were included in the study. We reviewed routine labs ordered by the IBD clinic including CBC, LFT, Lipid panel, and Iron panel. Abnormalities were defined as being outside of the normal reference range per Brown Health Laboratory guidelines. Data was securely stored using REDCap as part of an IRB-approved study. Descriptive statistics were performed using STATA MP18. Lab costs were estimated using the average of two major labs in RI and follow-up visits were based on average physician visit cost in RI.
Results: A total of 124 patients were analyzed in the study (Table 1). Over the study period, 817 labs were drawn ($45,279 total cost, $228 median per patient), 250 of which detected abnormalities (30.6%). However, only 15 (6%) of these abnormalities led to further workup (Table 2). One patient developed warm autoimmune hemolytic anemia, suspected related to Golimumab. However, the remainder of the lab abnormalities prompting further workup, costing another $55,310, were unrelated to their IBD medication.
Discussion: Our data suggests that laboratory surveillance monitoring may find abnormalities in about 1/4 tests, but these abnormalities may not be clinically related to their IBD medication and may be contributing to unnecessary healthcare cost. Further study is needed to guide clinicians on how frequently to monitor standard labs for IBD patients stable on their maintenance biologic or small molecule therapy.

Figure: Table 1: Study baseline patient characteristics

Figure: Abnormalities detected on routine lab monitoring and those that prompted further workup.
Disclosures:
Adam Burton indicated no relevant financial relationships.
Nicholas Scalzo indicated no relevant financial relationships.
Christina Raker indicated no relevant financial relationships.
Alex Chang indicated no relevant financial relationships.
Kimberly Ho indicated no relevant financial relationships.
Aaron Seto indicated no relevant financial relationships.
Cara Sammartino indicated no relevant financial relationships.
Sean Fine indicated no relevant financial relationships.
Adam M. Burton, MD1, Nicholas Scalzo, MD1, Christina Raker, MSc(Epi)2, Alex Chang, MD1, Kimberly Ho, MD1, Aaron Seto, BS3, Cara Sammartino, PhD, MSPH2, Sean Fine, MD1. P3284 - The Utility of Routine Laboratory Surveillance for Patients With Inflammatory Bowel Disease on Biologic or Small Molecule Therapy and Associated Costs, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
