Monday Poster Session
Category: IBD
P3407 - Intestinal Perforation in an Ulcerative Colitis Patient Following Upadicitinib Initiation
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Celeste R. Gracey, DO
University of Arizona College of Medicine
Tucson, AZ
Presenting Author(s)
Celeste R. Gracey, DO, Sasha Taleban, MD, FACG
University of Arizona College of Medicine, Tucson, AZ
Introduction: The non-selective Janus-Kinase (JAK) inhibitor, tofacitinib, has been associated with bowel perforation in ulcerative colitis (UC) patients. Upadacitinib (UPA), a selective JAK inhibitor, has rarely been associated with bowel perforation in Crohn’s disease (CD), and less commonly so in patients with UC. We present a rare case of bowel perforation in a UC patient three months after initiating UPA.
Case Description/
Methods: A 31-year female had a history of iron deficient anemia and UC for 9 years. After having failed or lost response to infliximab, azathioprine, vedolizumab, ozanimod, and ustekinumab, she started UPA with a significant improvement in fecal urgency and frequency of her bowel movements. Her C-reactive protein correspondingly improved from 18.1 to 5.3 mg/dL. She presented to an outside hospital with nausea, vomiting, and right lower quadrant tenderness. She had an elevated white blood cell count of 13.0x103 mcl as well as microcytic anemia with a hemoglobin of 11.0 g/dL. A computed tomography (CT) scan with intravenous contrast showed peri-colonic inflammation about the mid-transverse colon with extraluminal gas and posterior wall penetration without a drainable fluid collection. The left and right portions of the colon were decompressed with diffuse mucosal enhancement. She was not taking nonsteroidal anti-inflammatory agents or systemic corticosteroids for 4 months and had no history of perforations. The patient was conservatively managed with antibiotics and bowel rest, and her symptoms resolved over the course of a week. After follow up, the UPA was discontinued, and she was initiated on adalimumab.
Discussion: Prior investigations of perforation during UPA use in IBD reflect that the occurrences are so rare, it is difficult to determine whether they represent a true safety concern. The U-ACTIVATE UPA extension trial reported a colon perforation in a 70-year-old male. In the CD trials leading to approval of UPA, the U-EXCEL, U-EXCEED, and U-ENDURE reported 4 cases of perforation in the induction phase and 2 in the maintenance phase of the trials. In these cases, the patients had CD with active disease, deep ulcers, or strictures in the perforated areas, suggesting it might have been related to the natural course of their disease. In the case of our UC patient, her disease activity had improved in the months prior to her hospitalization. While rare, providers should consider discussing the risk of perforation in UC patients prior to initiation of UPA.
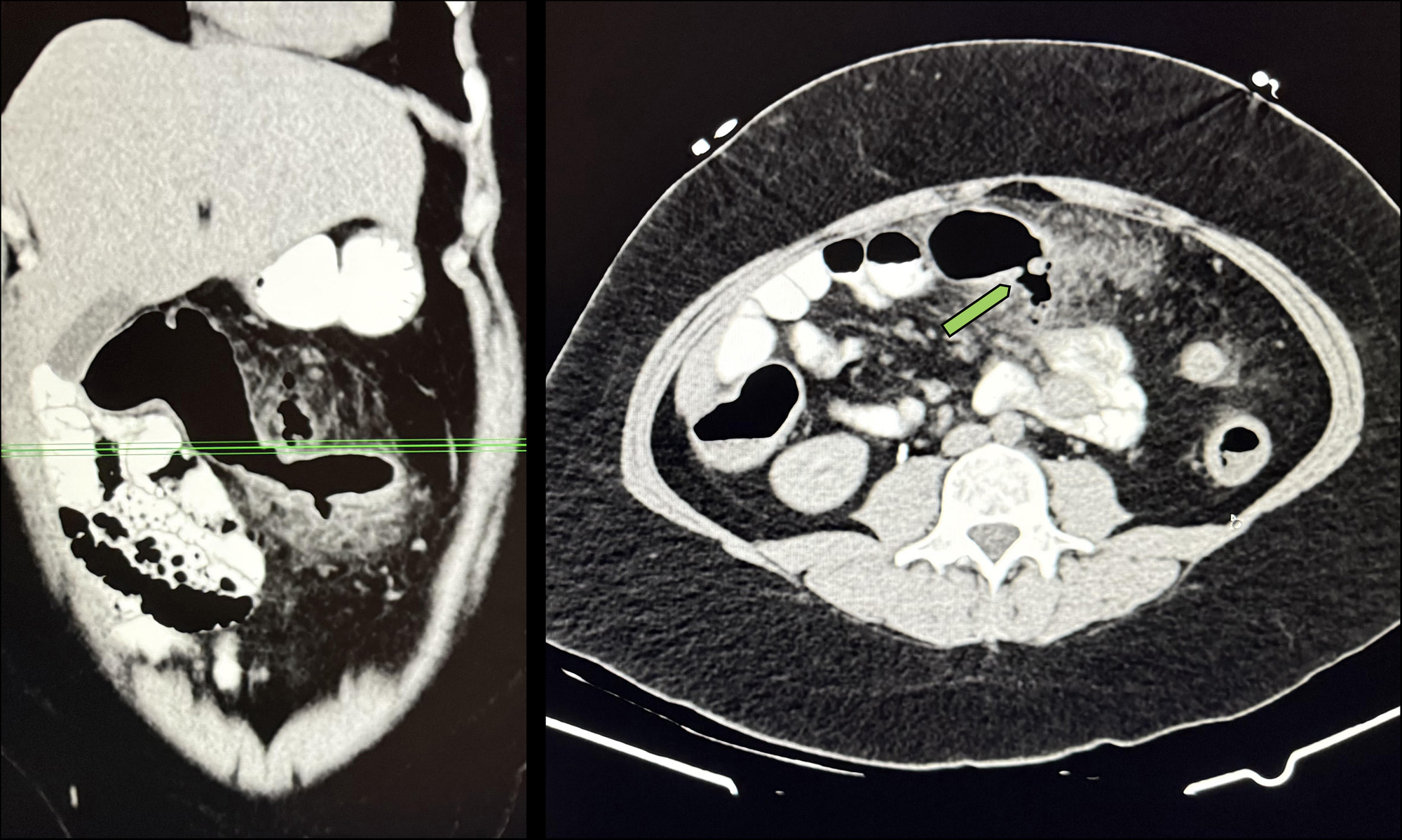
Figure: A computed tomography (CT) scan with intravenous contrast shows peri-colonic inflammation about the mid-transverse colon with extraluminal gas and posterior wall penetration without a drainable fluid collection. This image was captured from an ulcerative colitis patient three months following upadacitinib initiation.
Disclosures:
Celeste Gracey indicated no relevant financial relationships.
Sasha Taleban: AbbVie – Grant/Research Support. Boehringer-Ingelheim – Grant/Research Support. Eli Lilly – Grant/Research Support. Janssen – Advisory Committee/Board Member, Grant/Research Support. Pfizer – Grant/Research Support. Takeda – Grant/Research Support.
Celeste R. Gracey, DO, Sasha Taleban, MD, FACG. P3407 - Intestinal Perforation in an Ulcerative Colitis Patient Following Upadicitinib Initiation, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
University of Arizona College of Medicine, Tucson, AZ
Introduction: The non-selective Janus-Kinase (JAK) inhibitor, tofacitinib, has been associated with bowel perforation in ulcerative colitis (UC) patients. Upadacitinib (UPA), a selective JAK inhibitor, has rarely been associated with bowel perforation in Crohn’s disease (CD), and less commonly so in patients with UC. We present a rare case of bowel perforation in a UC patient three months after initiating UPA.
Case Description/
Methods: A 31-year female had a history of iron deficient anemia and UC for 9 years. After having failed or lost response to infliximab, azathioprine, vedolizumab, ozanimod, and ustekinumab, she started UPA with a significant improvement in fecal urgency and frequency of her bowel movements. Her C-reactive protein correspondingly improved from 18.1 to 5.3 mg/dL. She presented to an outside hospital with nausea, vomiting, and right lower quadrant tenderness. She had an elevated white blood cell count of 13.0x103 mcl as well as microcytic anemia with a hemoglobin of 11.0 g/dL. A computed tomography (CT) scan with intravenous contrast showed peri-colonic inflammation about the mid-transverse colon with extraluminal gas and posterior wall penetration without a drainable fluid collection. The left and right portions of the colon were decompressed with diffuse mucosal enhancement. She was not taking nonsteroidal anti-inflammatory agents or systemic corticosteroids for 4 months and had no history of perforations. The patient was conservatively managed with antibiotics and bowel rest, and her symptoms resolved over the course of a week. After follow up, the UPA was discontinued, and she was initiated on adalimumab.
Discussion: Prior investigations of perforation during UPA use in IBD reflect that the occurrences are so rare, it is difficult to determine whether they represent a true safety concern. The U-ACTIVATE UPA extension trial reported a colon perforation in a 70-year-old male. In the CD trials leading to approval of UPA, the U-EXCEL, U-EXCEED, and U-ENDURE reported 4 cases of perforation in the induction phase and 2 in the maintenance phase of the trials. In these cases, the patients had CD with active disease, deep ulcers, or strictures in the perforated areas, suggesting it might have been related to the natural course of their disease. In the case of our UC patient, her disease activity had improved in the months prior to her hospitalization. While rare, providers should consider discussing the risk of perforation in UC patients prior to initiation of UPA.

Figure: A computed tomography (CT) scan with intravenous contrast shows peri-colonic inflammation about the mid-transverse colon with extraluminal gas and posterior wall penetration without a drainable fluid collection. This image was captured from an ulcerative colitis patient three months following upadacitinib initiation.
Disclosures:
Celeste Gracey indicated no relevant financial relationships.
Sasha Taleban: AbbVie – Grant/Research Support. Boehringer-Ingelheim – Grant/Research Support. Eli Lilly – Grant/Research Support. Janssen – Advisory Committee/Board Member, Grant/Research Support. Pfizer – Grant/Research Support. Takeda – Grant/Research Support.
Celeste R. Gracey, DO, Sasha Taleban, MD, FACG. P3407 - Intestinal Perforation in an Ulcerative Colitis Patient Following Upadicitinib Initiation, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

