Monday Poster Session
Category: Interventional Endoscopy
P3557 - Underwater EMR Improves En-Bloc and Histologic Cure Rates Without Increasing Serious Adverse Events: A Systematic Review and Meta-Analysis of Three Comparative Trials
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
.jpg)
Ashesh Das, MBBS
KPC Medical College and Hospital , Kolkata, India
Kolkata, West Bengal, India
Presenting Author(s)
Ashesh Das, MBBS1, Venkata Dileep Kumar Veldi, MBBS2, Noorul Hidhaya, MBBS3, Daniel Razgonyaev, 4, Akif Shahid. Khan, MBBS5, Scott Tenner, MD6
1KPC Medical College and Hospital , Kolkata, India, Kolkata, West Bengal, India; 2Gayatri Vidya Parishad Institute of Health care and Medical Technology, Visakhapatnam, Andhra Pradesh, India; 3Stanley Medical College, Chennai, Tamil Nadu, India; 4SUNY Downstate Medical Center, Brooklyn, NY; 5Northwest School of Medicine, Nowshera, North-West Frontier, Pakistan; 6State University of New York, Downstate, Brooklyn, NY
Introduction: Conventional EMR remains the workhorse technique for large non-pedunculated colorectal polyps, yet piecemeal resection and positive margins drive recurrence rates up to 20 %. Under-water EMR (UEMR) eliminates submucosal injection, leverages the buoyant, collapsed lumen, and could therefore enhance en-bloc capture and histologic cure. While individual trials suggest technical superiority, the magnitude and consistency of UEMR’s benefit—and its impact on serious adverse events using only Randomized Controlled Trials (RCTs) have never been fully synthesized. We performed a focused meta-analysis of contemporary head-to-head RCTs to clarify UEMR’s true incremental value for colon polypectomy.
Methods: A systematic search of PubMed, Embase, Scopus, and Cochrane Library identified RCTs comparing Underwater EMR and Conventional EMR for Colonic Polyps through May 2025. Data were analysed using RevMan 4.2.1. Pooled risk ratios (RRs) with 95% confidence intervals (CIs) were calculated using Mantel-Haenszel methods. Random- or fixed-effects models were applied based on heterogeneity (Higgins’ I²). Statistical significance was set at p < 0.05. Risk of bias was assessed using RoB 2.0.
Results: Across three head-to-head trials comprising 574 colonic polyp resections, underwater EMR (UEMR) consistently out-performed conventional EMR (CEMR). UEMR achieved a higher en-bloc resection rate (64.7 % vs 56.3 %; RR 1.13, 95 % CI 1.02–1.26; p = 0.02) and superior histologic R0 resection (55.6 % vs 44.8 %; RR 1.22, 95 % CI 1.05–1.40; p = 0.008), with no heterogeneity detected (I² = 0 % for both). Although serious adverse events were numerically lower with UEMR (5.8 % vs 9.9 %; RR 0.61, 95 % CI 0.35–1.06; p = 0.08), the difference did not reach statistical significance.
Discussion: UEMR delivered statistically significant gains in both en-bloc and histologic R0 resection while numerically halving serious complications. The absence of heterogeneity strengthens confidence that these efficacy advantages are reproducible across practice settings. These data position UEMR as a compelling first-line strategy for large colorectal lesions, with a favorable—though not yet definitive—safety signal that warrants confirmation in larger multicenter studies. Adoption of UEMR could meaningfully advance complete, one-session colonic polypectomy and reduce the burden of surveillance and recurrence.
Figure: Figure Showing Forest Plot of En-Bloc Resection Rate, Histologic Complete Resection and Adverse Events
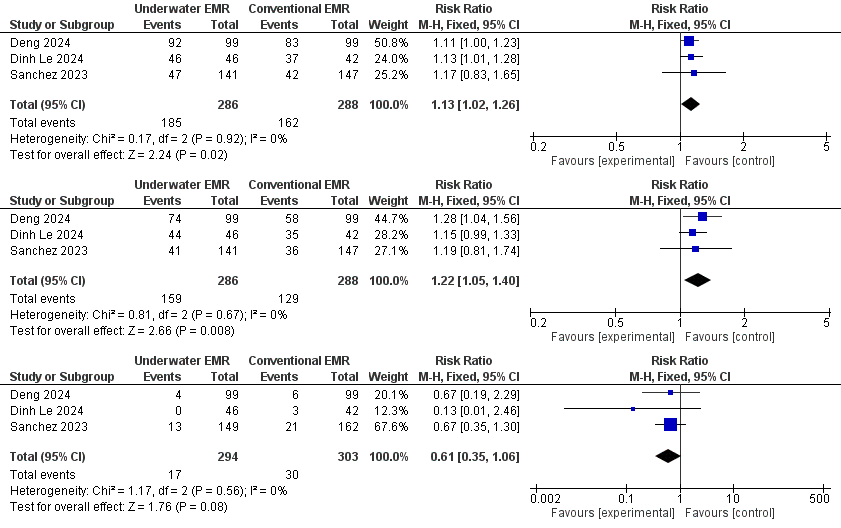
Figure: Prisma Flowchart
Disclosures:
Ashesh Das indicated no relevant financial relationships.
Venkata Dileep Kumar Veldi indicated no relevant financial relationships.
Noorul Hidhaya indicated no relevant financial relationships.
Daniel Razgonyaev indicated no relevant financial relationships.
Akif Khan indicated no relevant financial relationships.
Scott Tenner indicated no relevant financial relationships.
Ashesh Das, MBBS1, Venkata Dileep Kumar Veldi, MBBS2, Noorul Hidhaya, MBBS3, Daniel Razgonyaev, 4, Akif Shahid. Khan, MBBS5, Scott Tenner, MD6. P3557 - Underwater EMR Improves En-Bloc and Histologic Cure Rates Without Increasing Serious Adverse Events: A Systematic Review and Meta-Analysis of Three Comparative Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1KPC Medical College and Hospital , Kolkata, India, Kolkata, West Bengal, India; 2Gayatri Vidya Parishad Institute of Health care and Medical Technology, Visakhapatnam, Andhra Pradesh, India; 3Stanley Medical College, Chennai, Tamil Nadu, India; 4SUNY Downstate Medical Center, Brooklyn, NY; 5Northwest School of Medicine, Nowshera, North-West Frontier, Pakistan; 6State University of New York, Downstate, Brooklyn, NY
Introduction: Conventional EMR remains the workhorse technique for large non-pedunculated colorectal polyps, yet piecemeal resection and positive margins drive recurrence rates up to 20 %. Under-water EMR (UEMR) eliminates submucosal injection, leverages the buoyant, collapsed lumen, and could therefore enhance en-bloc capture and histologic cure. While individual trials suggest technical superiority, the magnitude and consistency of UEMR’s benefit—and its impact on serious adverse events using only Randomized Controlled Trials (RCTs) have never been fully synthesized. We performed a focused meta-analysis of contemporary head-to-head RCTs to clarify UEMR’s true incremental value for colon polypectomy.
Methods: A systematic search of PubMed, Embase, Scopus, and Cochrane Library identified RCTs comparing Underwater EMR and Conventional EMR for Colonic Polyps through May 2025. Data were analysed using RevMan 4.2.1. Pooled risk ratios (RRs) with 95% confidence intervals (CIs) were calculated using Mantel-Haenszel methods. Random- or fixed-effects models were applied based on heterogeneity (Higgins’ I²). Statistical significance was set at p < 0.05. Risk of bias was assessed using RoB 2.0.
Results: Across three head-to-head trials comprising 574 colonic polyp resections, underwater EMR (UEMR) consistently out-performed conventional EMR (CEMR). UEMR achieved a higher en-bloc resection rate (64.7 % vs 56.3 %; RR 1.13, 95 % CI 1.02–1.26; p = 0.02) and superior histologic R0 resection (55.6 % vs 44.8 %; RR 1.22, 95 % CI 1.05–1.40; p = 0.008), with no heterogeneity detected (I² = 0 % for both). Although serious adverse events were numerically lower with UEMR (5.8 % vs 9.9 %; RR 0.61, 95 % CI 0.35–1.06; p = 0.08), the difference did not reach statistical significance.
Discussion: UEMR delivered statistically significant gains in both en-bloc and histologic R0 resection while numerically halving serious complications. The absence of heterogeneity strengthens confidence that these efficacy advantages are reproducible across practice settings. These data position UEMR as a compelling first-line strategy for large colorectal lesions, with a favorable—though not yet definitive—safety signal that warrants confirmation in larger multicenter studies. Adoption of UEMR could meaningfully advance complete, one-session colonic polypectomy and reduce the burden of surveillance and recurrence.

Figure: Figure Showing Forest Plot of En-Bloc Resection Rate, Histologic Complete Resection and Adverse Events

Figure: Prisma Flowchart
Disclosures:
Ashesh Das indicated no relevant financial relationships.
Venkata Dileep Kumar Veldi indicated no relevant financial relationships.
Noorul Hidhaya indicated no relevant financial relationships.
Daniel Razgonyaev indicated no relevant financial relationships.
Akif Khan indicated no relevant financial relationships.
Scott Tenner indicated no relevant financial relationships.
Ashesh Das, MBBS1, Venkata Dileep Kumar Veldi, MBBS2, Noorul Hidhaya, MBBS3, Daniel Razgonyaev, 4, Akif Shahid. Khan, MBBS5, Scott Tenner, MD6. P3557 - Underwater EMR Improves En-Bloc and Histologic Cure Rates Without Increasing Serious Adverse Events: A Systematic Review and Meta-Analysis of Three Comparative Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
