Monday Poster Session
Category: Interventional Endoscopy
P3555 - Evaluating the Safety of GLP-1 Receptor Agonists in ERCP: A Propensity-Matched Analysis of Aspiration and Procedural Outcomes Before and After ASA Guideline Changes
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Mohammad AbuAssi, MD (he/him/his)
University of Central Florida, HCA Healthcare GME
Gainesville, FL
Presenting Author(s)
Mohammad Abuassi, MD1, Khaled Alsabbagh Alchirazi, MD2, Saqr Alsakarneh, MD, MS3, Kara Holt, MS4, Mohammed Janajri, MD5, Tony Brar, MD5, Yaseen Perbtani, DO1
1University of Central Florida, HCA Healthcare GME, Gainesville, FL; 2Aurora Health Care, Brookfield, WI; 3Mayo Clinic, Kansas City, MO; 4University of Florida College of Medicine, Gainesville, FL; 5University of Central Florida, Gainesville, FL
Introduction: GLP-1 receptor agonists (GLP-1 RAs) have become a cornerstone in managing type 2 diabetes and obesity but raise concerns regarding delayed gastric emptying and potential peri-procedural complications. Their safety in endoscopic retrograde cholangiopancreatography (ERCP), particularly regarding aspiration risk remains unclear, especially in light of updated ASA guidelines recommending pre-procedural withholding.
Methods: We used TriNetX, a federated network of de-identified electronic health records from U.S. healthcare systems, we identified adults who underwent ERCP with or without prior GLP-1 RA exposure (≥6 months of semaglutide, liraglutide, dulaglutide, or tirzepatide). Patients were matched 1:1 using propensity scores across 25 variables, including comorbidities and laboratory values. The primary outcome was aspiration pneumonitis within 7 days post-ERCP. Secondary outcomes included post-ERCP pancreatitis (PEP), ICU admission, emergency intubation, GI bleeding, perforation, and all-cause mortality. A sensitivity analysis compared GLP-1 RA users undergoing ERCP before vs. after the 2023 ASA guidelines.
Results: After propensity matching (n=3,218 per cohort), aspiration pneumonitis occurred in 0.3% of GLP-1 users compared to 0.5% of non-users (OR: 0.59; 95% CI: 0.27–1.28; p=0.177). GLP-1 use was associated with a significantly lower rate of ICU admission (3.6% vs. 4.8%; OR: 0.74; 95% CI: 0.58–0.95; p=0.018). Rates of post-ERCP pancreatitis (10.7% vs. 10.4%; p=0.746), GI bleeding, perforation, emergency intubation, and all-cause mortality were comparable between groups. In a sensitivity analysis, outcomes remained consistent when comparing GLP-1 users undergoing ERCP before versus after the 2023 ASA guideline update.
Discussion: GLP-1 RA use prior to ERCP was not associated with increased aspiration risk or adverse procedural outcomes. The consistency of outcomes before and after the ASA guideline update supports the peri-procedural safety of GLP-1 RAs and raises questions about the routine need to withhold therapy before endoscopic procedures.
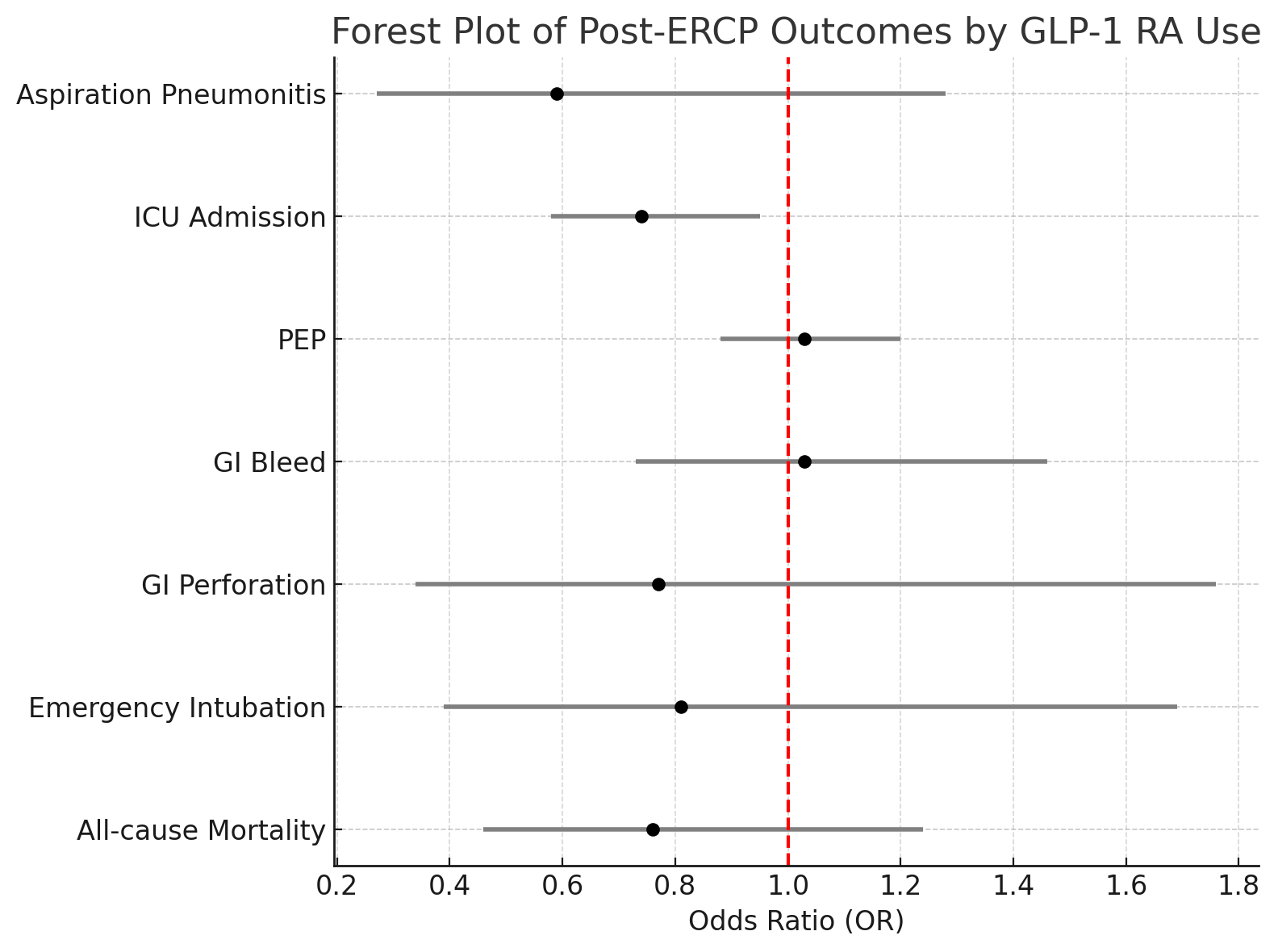
Figure: Forest plot of post-ERCP outcomes comparing GLP-1 receptor agonist users to matched non-users.
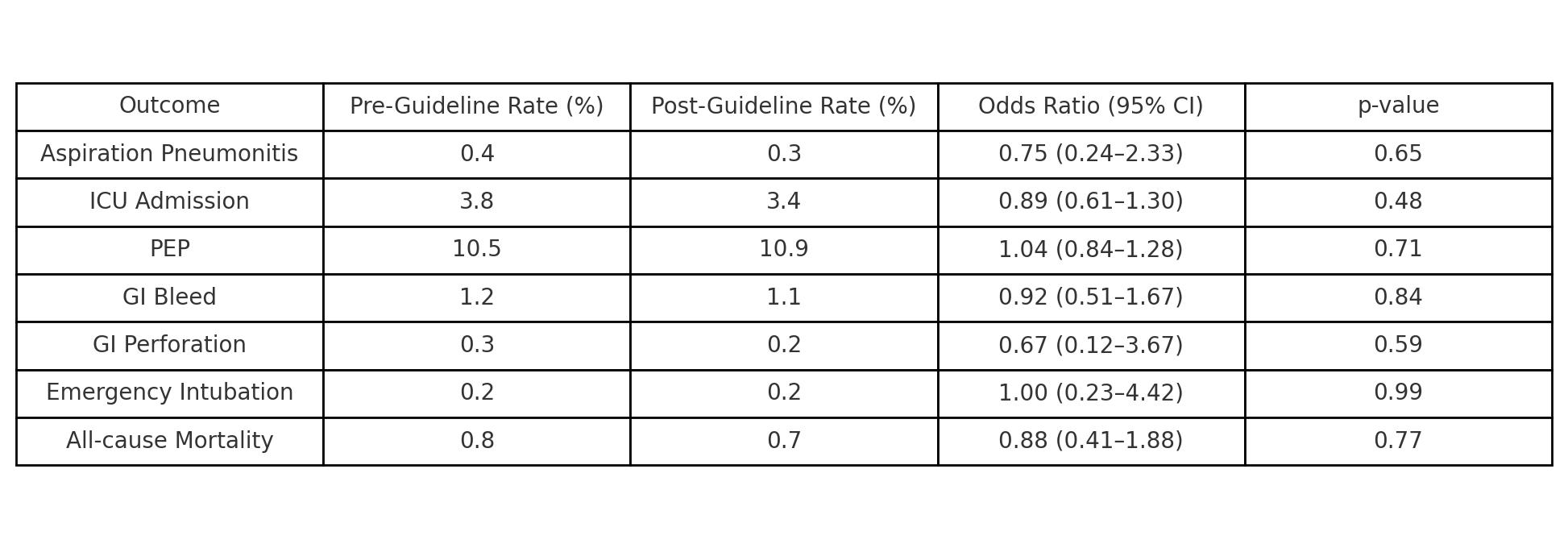
Figure: Comparison of post-ERCP outcomes in GLP-1 users before vs. after the 2023 ASA guideline update.
Disclosures:
Mohammad Abuassi indicated no relevant financial relationships.
Khaled Alsabbagh Alchirazi indicated no relevant financial relationships.
Saqr Alsakarneh indicated no relevant financial relationships.
Kara Holt indicated no relevant financial relationships.
Mohammed Janajri indicated no relevant financial relationships.
Tony Brar indicated no relevant financial relationships.
Yaseen Perbtani indicated no relevant financial relationships.
Mohammad Abuassi, MD1, Khaled Alsabbagh Alchirazi, MD2, Saqr Alsakarneh, MD, MS3, Kara Holt, MS4, Mohammed Janajri, MD5, Tony Brar, MD5, Yaseen Perbtani, DO1. P3555 - Evaluating the Safety of GLP-1 Receptor Agonists in ERCP: A Propensity-Matched Analysis of Aspiration and Procedural Outcomes Before and After ASA Guideline Changes, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Central Florida, HCA Healthcare GME, Gainesville, FL; 2Aurora Health Care, Brookfield, WI; 3Mayo Clinic, Kansas City, MO; 4University of Florida College of Medicine, Gainesville, FL; 5University of Central Florida, Gainesville, FL
Introduction: GLP-1 receptor agonists (GLP-1 RAs) have become a cornerstone in managing type 2 diabetes and obesity but raise concerns regarding delayed gastric emptying and potential peri-procedural complications. Their safety in endoscopic retrograde cholangiopancreatography (ERCP), particularly regarding aspiration risk remains unclear, especially in light of updated ASA guidelines recommending pre-procedural withholding.
Methods: We used TriNetX, a federated network of de-identified electronic health records from U.S. healthcare systems, we identified adults who underwent ERCP with or without prior GLP-1 RA exposure (≥6 months of semaglutide, liraglutide, dulaglutide, or tirzepatide). Patients were matched 1:1 using propensity scores across 25 variables, including comorbidities and laboratory values. The primary outcome was aspiration pneumonitis within 7 days post-ERCP. Secondary outcomes included post-ERCP pancreatitis (PEP), ICU admission, emergency intubation, GI bleeding, perforation, and all-cause mortality. A sensitivity analysis compared GLP-1 RA users undergoing ERCP before vs. after the 2023 ASA guidelines.
Results: After propensity matching (n=3,218 per cohort), aspiration pneumonitis occurred in 0.3% of GLP-1 users compared to 0.5% of non-users (OR: 0.59; 95% CI: 0.27–1.28; p=0.177). GLP-1 use was associated with a significantly lower rate of ICU admission (3.6% vs. 4.8%; OR: 0.74; 95% CI: 0.58–0.95; p=0.018). Rates of post-ERCP pancreatitis (10.7% vs. 10.4%; p=0.746), GI bleeding, perforation, emergency intubation, and all-cause mortality were comparable between groups. In a sensitivity analysis, outcomes remained consistent when comparing GLP-1 users undergoing ERCP before versus after the 2023 ASA guideline update.
Discussion: GLP-1 RA use prior to ERCP was not associated with increased aspiration risk or adverse procedural outcomes. The consistency of outcomes before and after the ASA guideline update supports the peri-procedural safety of GLP-1 RAs and raises questions about the routine need to withhold therapy before endoscopic procedures.

Figure: Forest plot of post-ERCP outcomes comparing GLP-1 receptor agonist users to matched non-users.

Figure: Comparison of post-ERCP outcomes in GLP-1 users before vs. after the 2023 ASA guideline update.
Disclosures:
Mohammad Abuassi indicated no relevant financial relationships.
Khaled Alsabbagh Alchirazi indicated no relevant financial relationships.
Saqr Alsakarneh indicated no relevant financial relationships.
Kara Holt indicated no relevant financial relationships.
Mohammed Janajri indicated no relevant financial relationships.
Tony Brar indicated no relevant financial relationships.
Yaseen Perbtani indicated no relevant financial relationships.
Mohammad Abuassi, MD1, Khaled Alsabbagh Alchirazi, MD2, Saqr Alsakarneh, MD, MS3, Kara Holt, MS4, Mohammed Janajri, MD5, Tony Brar, MD5, Yaseen Perbtani, DO1. P3555 - Evaluating the Safety of GLP-1 Receptor Agonists in ERCP: A Propensity-Matched Analysis of Aspiration and Procedural Outcomes Before and After ASA Guideline Changes, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
