Monday Poster Session
Category: Liver
P3632 - SGLT2 Inhibitors in the Management of Cirrhotic Ascites: A Meta-Analysis of Randomized Controlled Trials
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Gedion Yilma Amdetsion, MD (he/him/his)
Cook County Health
Chicago, IL
Presenting Author(s)
Gedion Yilma Amdetsion, MD1, Chun-wei Pan, MD1, Hiwot G. Tebeje, MD, MPH2, Shreyas Nandyal, MD1, Vikram Kotwal, MD3
1Cook County Health, Chicago, IL; 2Washington University in St. Louis, Saint Louis, MO; 3John H. Stroger, Jr. Hospital of Cook County, Chicago, IL
Introduction: Refractory ascites remains a major complication of decompensated cirrhosis, with limited effective pharmacologic options. Sodium-glucose cotransporter-2 (SGLT2) inhibitors are emerging as a novel class of agents with natriuretic and osmotic diuretic properties that may benefit patients with cirrhotic ascites.
Methods: We conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) evaluating SGLT2 inhibitors in adults with decompensated cirrhosis and refractory ascites. PubMed, Scopus, and Web of Science were searched through January 2025. Eligible studies included RCTs reporting risk ratios (RRs) or hazard ratios (HRs) for ascites-related outcomes. Meta-analyses were performed using fixed- or random-effects models based on heterogeneity. Study quality was assessed using the ROBINS-2 tool.
Results: Three RCTs comprising 110 patients met inclusion criteria. Compared to standard therapy, SGLT2 inhibitors significantly reduced the need for large-volume paracentesis (LVP) (HR = 0.56, 95% CI: 0.37–0.84, p = 0.005, I² = 0%) and nearly halved the proportion of patients requiring LVP (RR = 0.48, 95% CI: 0.33–0.70, p < 0.001). In two studies reporting complete ascites resolution, SGLT2 inhibitors were associated with a markedly increased likelihood of ultrasonographic clearance (RR = 9.00, 95% CI: 1.19–68.01, p = 0.03). No significant difference in acute kidney injury (AKI) was observed (RR = 1.98, 95% CI: 0.40–9.81, p = 0.40), though heterogeneity was high (I² = 78%). SGLT2 inhibitor use was associated with increased risks of urinary tract infection (RR = 3.00, 95% CI: 1.34–6.72, p = 0.01) and hyponatremia (RR = 2.80, 95% CI: 1.10–7.10, p = 0.03).
Discussion: SGLT2 inhibitors demonstrate promising efficacy in reducing the need for large-volume paracentesis and achieving ascites resolution in patients with refractory ascites secondary to cirrhosis. However, increased risks of urinary tract infections and hyponatremia warrant cautious patient selection and monitoring. Larger, multicenter trials with longer follow-up are needed to confirm these findings and further assess the safety profile of SGLT2 inhibitors in this population.
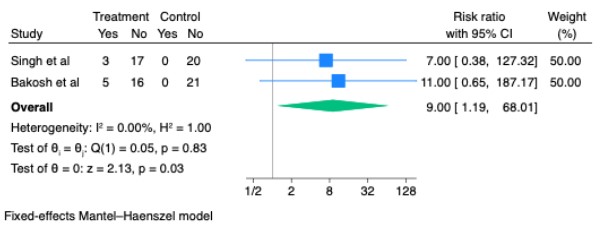
Figure: Risk Ratio for Complete Response with SGLT2 inhibitiors vs. Control in Included Studies
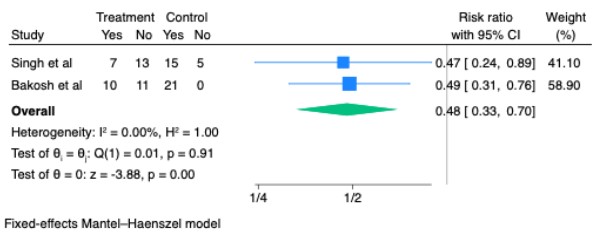
Figure: Risk Ratio for proportion of patients requiring LVP among those taking Treatment(SGLT2 inhibitions) vs. Control in Included Studies
Disclosures:
Gedion Yilma Amdetsion indicated no relevant financial relationships.
Chun-wei Pan indicated no relevant financial relationships.
Hiwot Tebeje indicated no relevant financial relationships.
Shreyas Nandyal indicated no relevant financial relationships.
Vikram Kotwal indicated no relevant financial relationships.
Gedion Yilma Amdetsion, MD1, Chun-wei Pan, MD1, Hiwot G. Tebeje, MD, MPH2, Shreyas Nandyal, MD1, Vikram Kotwal, MD3. P3632 - SGLT2 Inhibitors in the Management of Cirrhotic Ascites: A Meta-Analysis of Randomized Controlled Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Cook County Health, Chicago, IL; 2Washington University in St. Louis, Saint Louis, MO; 3John H. Stroger, Jr. Hospital of Cook County, Chicago, IL
Introduction: Refractory ascites remains a major complication of decompensated cirrhosis, with limited effective pharmacologic options. Sodium-glucose cotransporter-2 (SGLT2) inhibitors are emerging as a novel class of agents with natriuretic and osmotic diuretic properties that may benefit patients with cirrhotic ascites.
Methods: We conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) evaluating SGLT2 inhibitors in adults with decompensated cirrhosis and refractory ascites. PubMed, Scopus, and Web of Science were searched through January 2025. Eligible studies included RCTs reporting risk ratios (RRs) or hazard ratios (HRs) for ascites-related outcomes. Meta-analyses were performed using fixed- or random-effects models based on heterogeneity. Study quality was assessed using the ROBINS-2 tool.
Results: Three RCTs comprising 110 patients met inclusion criteria. Compared to standard therapy, SGLT2 inhibitors significantly reduced the need for large-volume paracentesis (LVP) (HR = 0.56, 95% CI: 0.37–0.84, p = 0.005, I² = 0%) and nearly halved the proportion of patients requiring LVP (RR = 0.48, 95% CI: 0.33–0.70, p < 0.001). In two studies reporting complete ascites resolution, SGLT2 inhibitors were associated with a markedly increased likelihood of ultrasonographic clearance (RR = 9.00, 95% CI: 1.19–68.01, p = 0.03). No significant difference in acute kidney injury (AKI) was observed (RR = 1.98, 95% CI: 0.40–9.81, p = 0.40), though heterogeneity was high (I² = 78%). SGLT2 inhibitor use was associated with increased risks of urinary tract infection (RR = 3.00, 95% CI: 1.34–6.72, p = 0.01) and hyponatremia (RR = 2.80, 95% CI: 1.10–7.10, p = 0.03).
Discussion: SGLT2 inhibitors demonstrate promising efficacy in reducing the need for large-volume paracentesis and achieving ascites resolution in patients with refractory ascites secondary to cirrhosis. However, increased risks of urinary tract infections and hyponatremia warrant cautious patient selection and monitoring. Larger, multicenter trials with longer follow-up are needed to confirm these findings and further assess the safety profile of SGLT2 inhibitors in this population.

Figure: Risk Ratio for Complete Response with SGLT2 inhibitiors vs. Control in Included Studies

Figure: Risk Ratio for proportion of patients requiring LVP among those taking Treatment(SGLT2 inhibitions) vs. Control in Included Studies
Disclosures:
Gedion Yilma Amdetsion indicated no relevant financial relationships.
Chun-wei Pan indicated no relevant financial relationships.
Hiwot Tebeje indicated no relevant financial relationships.
Shreyas Nandyal indicated no relevant financial relationships.
Vikram Kotwal indicated no relevant financial relationships.
Gedion Yilma Amdetsion, MD1, Chun-wei Pan, MD1, Hiwot G. Tebeje, MD, MPH2, Shreyas Nandyal, MD1, Vikram Kotwal, MD3. P3632 - SGLT2 Inhibitors in the Management of Cirrhotic Ascites: A Meta-Analysis of Randomized Controlled Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
