Monday Poster Session
Category: Liver
P3781 - Predictors of Undetectable Hepatitis Delta Virus RNA at 48 Weeks After End of Treatment With Bulevirtide Monotherapy in the MYR 301 Study
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Renee-Claude Mercier, PharmD
Gilead Sciences, Inc.
Foster City, CA
Presenting Author(s)
Soo Aleman, MD, PhD1, Maurizia R. Brunetto, MD2, Antje Blank, MD3, Pietro Andreone, MD4, Pavel Bogomolov, MD, PhD5, Vladimir Chulanov, MD, PhD, DSc6, Nina Mamonova, MD7, Natalia Geyvandova, MD8, Viacheslav Morozov, MD, PhD9, Olga Sagalova, PhD10, Tatiana Stepanova, MD11, Amos Lichtman, MD, MPH12, Renee-Claude Mercier, PharmD12, Dmitry Manuilov, MD12, Sarah Arterburn, MS12, Julian Schulze zur Wiesch, MD13, Markus Cornberg, MD14, Stefan Zeuzem, MD15, Pietro Lampertico, MD, PhD16, Heiner Wedemeyer, MD14
1Department of Infectious Diseases, Karolinska University Hospital/Karolinska Institutet, Stockholm, Stockholms Lan, Sweden; 2Hepatology Unit, Reference Center of the Tuscany Region for Chronic Liver Disease and Cancer, University Hospital of Pisa; Department of Clinical and Experimental Medicine, University of Pisa, Pisa, Toscana, Italy; 3Medical Faculty Heidelberg/Heidelberg University Hospital, Department of Clinical Pharmacology and Pharmacoepidemiology, Heidelberg, Baden-Wurttemberg, Germany; 4Department of Internal Medicine, Baggiovara Hospital, University of Modena and Reggio Emilia, Modena, Italy, Modena, Emilia-Romagna, Italy; 5M.F. Vladimirsky Moscow Regional Research and Clinical Institute, Moscow, Moskva, Russia; 6Sechenov University, Moscow, Moskva, Russia; 7FSBI National Research Medical Center for Phthisiopulmonology and Infectious Diseases of the Ministry of Health of the Russian Federation, Moscow, Moskva, Russia; 8Stavropol Regional Hospital, Stavropol, Stavropol', Russia; 9LLC Medical Company “Hepatolog”, Samara, Samara, Russia; 10South Ural State Medical University, Chelyabinsk, Orel, Russia; 11LLC Clinic of Modern Medicine, Moscow, Moskva, Russia; 12Gilead Sciences, Inc., Foster City, CA; 13Hepatology Outpatient Medical Clinic, University Hospital Hamburg-Eppendorf, Hamburg, Hamburg, Germany; 14Clinic for Gastroenterology, Hepatology, Infectious Diseases, and Endocrinology, Hannover Medical School, Hannover, Sachsen, Germany; 15Department of Medicine, University Hospital, Goethe University Frankfurt, Frankfurt, Hessen, Germany; 16Division of Gastroenterology and Hepatology, Foundation IRCCS Ca’ Granda Ospedale Maggiore Policlinico; CRC “A. M. and A. Migliavacca” Center for Liver Disease, Department of Pathophysiology and Transplantation, University of Milan, Milan, Lombardia, Italy
Introduction: In the MYR301 Phase 3 study, a subset of patients (pts) with chronic hepatitis delta (CHD) receiving bulevirtide (BLV) monotherapy achieved undetectable HDV RNA by end of treatment (EOT, 2–3 years) and maintained undetectable viremia at 48 weeks (W) after EOT (FU48). We present predictors of sustained HDV RNA undetectability through FU48 after 96W or 144W of BLV treatment.
Methods: Pts with CHD in MYR301 (n=149) were randomized to immediate treatment with BLV 2 or 10 mg/d for 144W, or delayed treatment for 48W followed by BLV 10 mg/d for 96W (DT/10 mg) and followed through FU48. Logistic regression modelling (adjusted for treatment group) was evaluated via odds ratios (OR [95% CI]) for potential predictors of sustained HDV RNA undetectability (defined as < the lower limit of quantitation [LLOQ; target not detected at FU]) through FU48 in pts with undetectable viremia at EOT.
Results: Baseline (BL) characteristics were similar between treatments. Overall, 65/149 (44%) pts achieved HDV RNA undetectability at EOT; 23/64 (36%) with FU data sustained undetectability through FU48. Sustained undetectability rates were higher in the immediate treatment arms (2 mg: 7/14, 50%; 10 mg: 10/25, 40%) vs the DT/10 mg (6/26, 23%) arm. Undetectability rates at EOT were higher in pts receiving BLV 10 mg; the relapse rate was lower in pts with HDV RNA < LLOQ in the BLV 2 mg group. BL predictors of sustained undetectability posttreatment (PT) included BL median HDV RNA < 4.5 log10 IU/mL (OR [95% CI]: 6.2 [1.9, 20.8]; P=.003) and lower BL hepatitis B surface antigen (HBsAg) level (0.3 per log10 IU/mL [0.1, 0.8]; P=.019). On-treatment predictors included greater duration of continuous undetectability at EOT (per additional week: 1.0 [1.0, 1.1]; P< .0001), HBsAg loss/decrease by ≥1 log10 IU/mL (7.2 [1.2, 42.3]; P=.030), and W144 antidrug antibody (ADA) incidence (10.2 [1.9, 55.7]; P=.008). The proportion of pts with sustained PT undetectability was 9/10 (90%) in pts with ≥96W of undetectability at EOT, 11/22 (50%) in pts with ≥48 to < 96W, and 3/32 (9%) in pts with < 48W. Of pts with ADA incidence by W144, 8/10 (80%) had sustained undetectability vs 15/54 (28%) of pts without. BL cirrhosis was not a predictor of sustained PT undetectability (13/32 [41%] with vs 10/32 [31%] without cirrhosis).
Discussion: In pts with CHD treated with BLV monotherapy for 96W or 144W, early and sustained HDV RNA undetectability predicted undetectability at EOT and sustained undetectability during FU. PL and HW contributed equally.
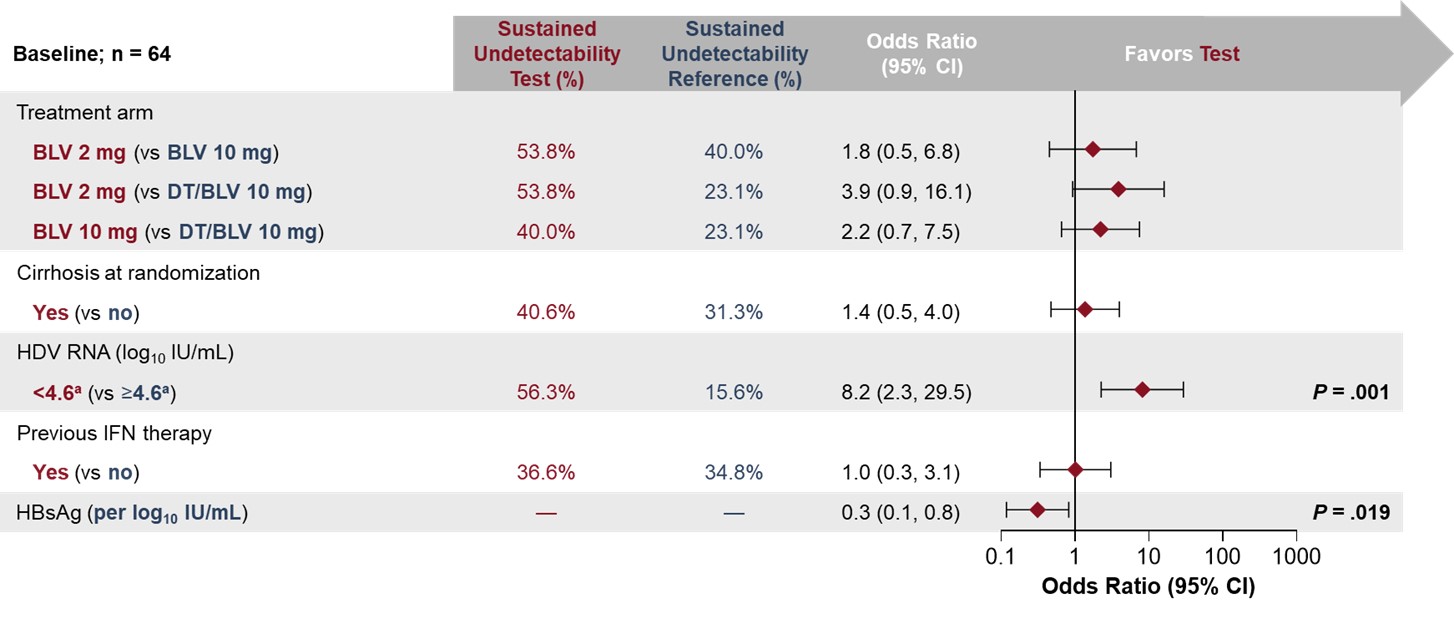
Figure: Figure 1. Baseline predictors of sustained HDV RNA undetectability after EOT (FU48)
aRepresents the median.
BLV, bulevirtide; DT, delayed treatment; EOT, end of treatment; FU48, follow-up at 48 weeks after EOT (week 192); HBsAg, hepatitis B surface antigen; HDV, hepatitis delta virus; IFN, interferon.
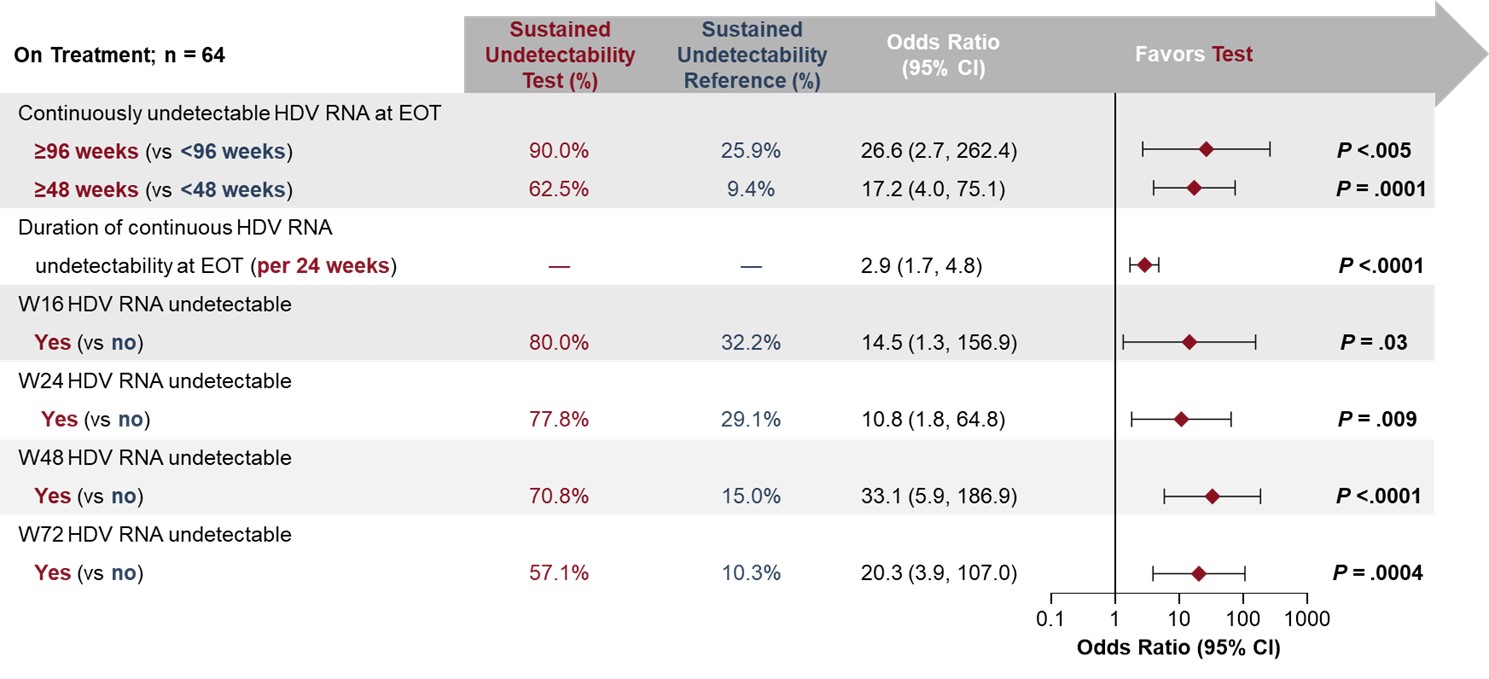
Figure: Figure 2. On-treatment predictors of sustained HDV RNA undetectability after EOT (FU48)
Bold P-values indicate significance.
EOT, end of treatment; FU48, follow-up at 48 weeks after EOT (week 192); HDV, hepatitis delta virus; W, week.
Disclosures:
Soo Aleman: AbbVie – Honoraria for educational events, Speakers Bureau. Biogen – Honoraria for educational events, Speakers Bureau. Gilead Sciences, Inc. – Honoraria for educational events, Speakers Bureau. GSK – Honoraria for educational events, Speakers Bureau. Janssen – Honoraria for educational events, Speakers Bureau. Merck Sharp & Dohme – Honoraria for educational events, Speakers Bureau. MYR GmbH – Honoraria for educational events, Speakers Bureau. Roche – Honoraria for educational events, Speakers Bureau. Spring Bank Pharmaceuticals – Honoraria for educational events, Speakers Bureau.
Maurizia Brunetto: AbbVie – Consultant, Speakers Bureau. AstraZeneca – Consultant, Speakers Bureau. Gilead Sciences, Inc. – Consultant, Speakers Bureau. Janssen – Consultant, Speakers Bureau. Merck Sharp & Dohme-Eisai – Consultant, Speakers Bureau. Roche – Consultant, Speakers Bureau.
Antje Blank: Gilead Sciences, Inc. – Research funding. MYR GmbH – Research funding.
Pietro Andreone indicated no relevant financial relationships.
Pavel Bogomolov: AbbVie – Grant/Research Support, Speakers Bureau. Bayer – Grant/Research Support, Speakers Bureau. Gilead Sciences, Inc. – Grant/Research Support, Speakers Bureau. Hepatera – Grant/Research Support, Speakers Bureau. Merck Sharp & Dohme – Grant/Research Support, Speakers Bureau. Norvo Nordisk – Grant/Research Support, Speakers Bureau. R-Pharm – Grant/Research Support, Speakers Bureau.
Vladimir Chulanov: AbbVie – Consultant, Speakers Bureau. AstraZeneca – Consultant, Speakers Bureau. Bristol Myers Squibb – Consultant, Speakers Bureau. Gilead Sciences, Inc. – Consultant, Speakers Bureau. GSK – Consultant, Speakers Bureau. Hepatera – Consultant, Speakers Bureau. Merck Sharp & Dohme – Consultant, Speakers Bureau. Roche – Consultant, Speakers Bureau. R-Pharm – Consultant, Speakers Bureau.
Nina Mamonova indicated no relevant financial relationships.
Natalia Geyvandova indicated no relevant financial relationships.
Viacheslav Morozov indicated no relevant financial relationships.
Olga Sagalova indicated no relevant financial relationships.
Tatiana Stepanova indicated no relevant financial relationships.
Amos Lichtman: Gilead Sciences, Inc. – Employee, Stock Options.
Renee-Claude Mercier: Gilead Sciences, Inc. – Employee, Stock Options.
Dmitry Manuilov: Gilead Sciences, Inc. – Employee, Stock Options.
Sarah Arterburn: Gilead Sciences, Inc. – Employee, Stock Options.
Julian Schulze zur Wiesch: Gilead Sciences, Inc. – Consultant.
Markus Cornberg: AbbVie – Honoraria. Falk – Honoraria. Gilead Sciences, Inc. – Honoraria. GSK – Honoraria. Janssen-Cilag – Honoraria. Merck Sharp & Dohme – Honoraria. Novartis – Honoraria. Roche – Honoraria. Spring Bank Pharmaceuticals – Honoraria. Swedish Orphan Biovitrum – Honoraria.
Stefan Zeuzem indicated no relevant financial relationships.
Pietro Lampertico: AbbVie – Advisory Committee/Board Member, Speakers Bureau. Aligos Therapeutics – Advisory Committee/Board Member, Speakers Bureau. Alnylam Pharmaceuticals – Advisory Committee/Board Member, Speakers Bureau. Antios Therapeutics – Advisory Committee/Board Member, Speakers Bureau. Arrowhead Pharmaceuticals – Advisory Committee/Board Member, Speakers Bureau. Bristol Myers Squibb – Advisory Committee/Board Member, Speakers Bureau. Eiger Pharmaceuticals – Advisory Committee/Board Member, Speakers Bureau. Gilead Sciences, Inc. – Advisory Committee/Board Member, Speakers Bureau. GSK – Advisory Committee/Board Member, Speakers Bureau. Janssen – Advisory Committee/Board Member, Speakers Bureau. Merck Sharp & Dohme – Advisory Committee/Board Member, Speakers Bureau. MYR GmbH – Advisory Committee/Board Member, Speakers Bureau. Roche – Advisory Committee/Board Member, Speakers Bureau. Spring Bank Pharmaceuticals – Advisory Committee/Board Member, Speakers Bureau.
Heiner Wedemeyer: Abbott Laboratories – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Abbott Molecular – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Albireo Pharma – Advisory Committee/Board Member, Consultant, Speakers Bureau. AstraZeneca – Advisory Committee/Board Member, Consultant, Speakers Bureau. Atea Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speakers Bureau. BioMarin Pharmaceutical – Advisory Committee/Board Member, Consultant, Speakers Bureau. Biotest – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speakers Bureau. CSL Behring – Advisory Committee/Board Member, Consultant, Speakers Bureau. Dr. Falk Pharma – Advisory Committee/Board Member, Consultant, Speakers Bureau. Falk Foundation – Advisory Committee/Board Member, Consultant, Speakers Bureau. Gilead Sciences, Inc. – Advisory Committee/Board Member, Consultant, Speakers Bureau. GSK – Advisory Committee/Board Member, Consultant, Speakers Bureau. Janssen – Advisory Committee/Board Member, Consultant, Speakers Bureau. Lilly Deutschland – Advisory Committee/Board Member, Consultant, Speakers Bureau. Merck Sharp & Dohme – Advisory Committee/Board Member, Consultant, Speakers Bureau. Mirum Pharma Germany – Advisory Committee/Board Member, Consultant, Speakers Bureau. Olink – Advisory Committee/Board Member, Consultant, Speakers Bureau. Orphalan – Advisory Committee/Board Member, Consultant, Speakers Bureau. Pfizer – Advisory Committee/Board Member, Consultant, Speakers Bureau. Roche – Advisory Committee/Board Member, Consultant, Speakers Bureau. Roche Diagnostics International – Advisory Committee/Board Member, Consultant, Speakers Bureau. Roche Pharma – Advisory Committee/Board Member, Consultant, Speakers Bureau. Swedish Orphan Biovitrum – Advisory Committee/Board Member, Consultant, Speakers Bureau. Takeda Pharma Vertrieb – Advisory Committee/Board Member, Consultant, Speakers Bureau. Vir Biotechnology – Advisory Committee/Board Member, Consultant, Speakers Bureau.
Soo Aleman, MD, PhD1, Maurizia R. Brunetto, MD2, Antje Blank, MD3, Pietro Andreone, MD4, Pavel Bogomolov, MD, PhD5, Vladimir Chulanov, MD, PhD, DSc6, Nina Mamonova, MD7, Natalia Geyvandova, MD8, Viacheslav Morozov, MD, PhD9, Olga Sagalova, PhD10, Tatiana Stepanova, MD11, Amos Lichtman, MD, MPH12, Renee-Claude Mercier, PharmD12, Dmitry Manuilov, MD12, Sarah Arterburn, MS12, Julian Schulze zur Wiesch, MD13, Markus Cornberg, MD14, Stefan Zeuzem, MD15, Pietro Lampertico, MD, PhD16, Heiner Wedemeyer, MD14. P3781 - Predictors of Undetectable Hepatitis Delta Virus RNA at 48 Weeks After End of Treatment With Bulevirtide Monotherapy in the MYR 301 Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Department of Infectious Diseases, Karolinska University Hospital/Karolinska Institutet, Stockholm, Stockholms Lan, Sweden; 2Hepatology Unit, Reference Center of the Tuscany Region for Chronic Liver Disease and Cancer, University Hospital of Pisa; Department of Clinical and Experimental Medicine, University of Pisa, Pisa, Toscana, Italy; 3Medical Faculty Heidelberg/Heidelberg University Hospital, Department of Clinical Pharmacology and Pharmacoepidemiology, Heidelberg, Baden-Wurttemberg, Germany; 4Department of Internal Medicine, Baggiovara Hospital, University of Modena and Reggio Emilia, Modena, Italy, Modena, Emilia-Romagna, Italy; 5M.F. Vladimirsky Moscow Regional Research and Clinical Institute, Moscow, Moskva, Russia; 6Sechenov University, Moscow, Moskva, Russia; 7FSBI National Research Medical Center for Phthisiopulmonology and Infectious Diseases of the Ministry of Health of the Russian Federation, Moscow, Moskva, Russia; 8Stavropol Regional Hospital, Stavropol, Stavropol', Russia; 9LLC Medical Company “Hepatolog”, Samara, Samara, Russia; 10South Ural State Medical University, Chelyabinsk, Orel, Russia; 11LLC Clinic of Modern Medicine, Moscow, Moskva, Russia; 12Gilead Sciences, Inc., Foster City, CA; 13Hepatology Outpatient Medical Clinic, University Hospital Hamburg-Eppendorf, Hamburg, Hamburg, Germany; 14Clinic for Gastroenterology, Hepatology, Infectious Diseases, and Endocrinology, Hannover Medical School, Hannover, Sachsen, Germany; 15Department of Medicine, University Hospital, Goethe University Frankfurt, Frankfurt, Hessen, Germany; 16Division of Gastroenterology and Hepatology, Foundation IRCCS Ca’ Granda Ospedale Maggiore Policlinico; CRC “A. M. and A. Migliavacca” Center for Liver Disease, Department of Pathophysiology and Transplantation, University of Milan, Milan, Lombardia, Italy
Introduction: In the MYR301 Phase 3 study, a subset of patients (pts) with chronic hepatitis delta (CHD) receiving bulevirtide (BLV) monotherapy achieved undetectable HDV RNA by end of treatment (EOT, 2–3 years) and maintained undetectable viremia at 48 weeks (W) after EOT (FU48). We present predictors of sustained HDV RNA undetectability through FU48 after 96W or 144W of BLV treatment.
Methods: Pts with CHD in MYR301 (n=149) were randomized to immediate treatment with BLV 2 or 10 mg/d for 144W, or delayed treatment for 48W followed by BLV 10 mg/d for 96W (DT/10 mg) and followed through FU48. Logistic regression modelling (adjusted for treatment group) was evaluated via odds ratios (OR [95% CI]) for potential predictors of sustained HDV RNA undetectability (defined as < the lower limit of quantitation [LLOQ; target not detected at FU]) through FU48 in pts with undetectable viremia at EOT.
Results: Baseline (BL) characteristics were similar between treatments. Overall, 65/149 (44%) pts achieved HDV RNA undetectability at EOT; 23/64 (36%) with FU data sustained undetectability through FU48. Sustained undetectability rates were higher in the immediate treatment arms (2 mg: 7/14, 50%; 10 mg: 10/25, 40%) vs the DT/10 mg (6/26, 23%) arm. Undetectability rates at EOT were higher in pts receiving BLV 10 mg; the relapse rate was lower in pts with HDV RNA < LLOQ in the BLV 2 mg group. BL predictors of sustained undetectability posttreatment (PT) included BL median HDV RNA < 4.5 log10 IU/mL (OR [95% CI]: 6.2 [1.9, 20.8]; P=.003) and lower BL hepatitis B surface antigen (HBsAg) level (0.3 per log10 IU/mL [0.1, 0.8]; P=.019). On-treatment predictors included greater duration of continuous undetectability at EOT (per additional week: 1.0 [1.0, 1.1]; P< .0001), HBsAg loss/decrease by ≥1 log10 IU/mL (7.2 [1.2, 42.3]; P=.030), and W144 antidrug antibody (ADA) incidence (10.2 [1.9, 55.7]; P=.008). The proportion of pts with sustained PT undetectability was 9/10 (90%) in pts with ≥96W of undetectability at EOT, 11/22 (50%) in pts with ≥48 to < 96W, and 3/32 (9%) in pts with < 48W. Of pts with ADA incidence by W144, 8/10 (80%) had sustained undetectability vs 15/54 (28%) of pts without. BL cirrhosis was not a predictor of sustained PT undetectability (13/32 [41%] with vs 10/32 [31%] without cirrhosis).
Discussion: In pts with CHD treated with BLV monotherapy for 96W or 144W, early and sustained HDV RNA undetectability predicted undetectability at EOT and sustained undetectability during FU. PL and HW contributed equally.

Figure: Figure 1. Baseline predictors of sustained HDV RNA undetectability after EOT (FU48)
aRepresents the median.
BLV, bulevirtide; DT, delayed treatment; EOT, end of treatment; FU48, follow-up at 48 weeks after EOT (week 192); HBsAg, hepatitis B surface antigen; HDV, hepatitis delta virus; IFN, interferon.

Figure: Figure 2. On-treatment predictors of sustained HDV RNA undetectability after EOT (FU48)
Bold P-values indicate significance.
EOT, end of treatment; FU48, follow-up at 48 weeks after EOT (week 192); HDV, hepatitis delta virus; W, week.
Disclosures:
Soo Aleman: AbbVie – Honoraria for educational events, Speakers Bureau. Biogen – Honoraria for educational events, Speakers Bureau. Gilead Sciences, Inc. – Honoraria for educational events, Speakers Bureau. GSK – Honoraria for educational events, Speakers Bureau. Janssen – Honoraria for educational events, Speakers Bureau. Merck Sharp & Dohme – Honoraria for educational events, Speakers Bureau. MYR GmbH – Honoraria for educational events, Speakers Bureau. Roche – Honoraria for educational events, Speakers Bureau. Spring Bank Pharmaceuticals – Honoraria for educational events, Speakers Bureau.
Maurizia Brunetto: AbbVie – Consultant, Speakers Bureau. AstraZeneca – Consultant, Speakers Bureau. Gilead Sciences, Inc. – Consultant, Speakers Bureau. Janssen – Consultant, Speakers Bureau. Merck Sharp & Dohme-Eisai – Consultant, Speakers Bureau. Roche – Consultant, Speakers Bureau.
Antje Blank: Gilead Sciences, Inc. – Research funding. MYR GmbH – Research funding.
Pietro Andreone indicated no relevant financial relationships.
Pavel Bogomolov: AbbVie – Grant/Research Support, Speakers Bureau. Bayer – Grant/Research Support, Speakers Bureau. Gilead Sciences, Inc. – Grant/Research Support, Speakers Bureau. Hepatera – Grant/Research Support, Speakers Bureau. Merck Sharp & Dohme – Grant/Research Support, Speakers Bureau. Norvo Nordisk – Grant/Research Support, Speakers Bureau. R-Pharm – Grant/Research Support, Speakers Bureau.
Vladimir Chulanov: AbbVie – Consultant, Speakers Bureau. AstraZeneca – Consultant, Speakers Bureau. Bristol Myers Squibb – Consultant, Speakers Bureau. Gilead Sciences, Inc. – Consultant, Speakers Bureau. GSK – Consultant, Speakers Bureau. Hepatera – Consultant, Speakers Bureau. Merck Sharp & Dohme – Consultant, Speakers Bureau. Roche – Consultant, Speakers Bureau. R-Pharm – Consultant, Speakers Bureau.
Nina Mamonova indicated no relevant financial relationships.
Natalia Geyvandova indicated no relevant financial relationships.
Viacheslav Morozov indicated no relevant financial relationships.
Olga Sagalova indicated no relevant financial relationships.
Tatiana Stepanova indicated no relevant financial relationships.
Amos Lichtman: Gilead Sciences, Inc. – Employee, Stock Options.
Renee-Claude Mercier: Gilead Sciences, Inc. – Employee, Stock Options.
Dmitry Manuilov: Gilead Sciences, Inc. – Employee, Stock Options.
Sarah Arterburn: Gilead Sciences, Inc. – Employee, Stock Options.
Julian Schulze zur Wiesch: Gilead Sciences, Inc. – Consultant.
Markus Cornberg: AbbVie – Honoraria. Falk – Honoraria. Gilead Sciences, Inc. – Honoraria. GSK – Honoraria. Janssen-Cilag – Honoraria. Merck Sharp & Dohme – Honoraria. Novartis – Honoraria. Roche – Honoraria. Spring Bank Pharmaceuticals – Honoraria. Swedish Orphan Biovitrum – Honoraria.
Stefan Zeuzem indicated no relevant financial relationships.
Pietro Lampertico: AbbVie – Advisory Committee/Board Member, Speakers Bureau. Aligos Therapeutics – Advisory Committee/Board Member, Speakers Bureau. Alnylam Pharmaceuticals – Advisory Committee/Board Member, Speakers Bureau. Antios Therapeutics – Advisory Committee/Board Member, Speakers Bureau. Arrowhead Pharmaceuticals – Advisory Committee/Board Member, Speakers Bureau. Bristol Myers Squibb – Advisory Committee/Board Member, Speakers Bureau. Eiger Pharmaceuticals – Advisory Committee/Board Member, Speakers Bureau. Gilead Sciences, Inc. – Advisory Committee/Board Member, Speakers Bureau. GSK – Advisory Committee/Board Member, Speakers Bureau. Janssen – Advisory Committee/Board Member, Speakers Bureau. Merck Sharp & Dohme – Advisory Committee/Board Member, Speakers Bureau. MYR GmbH – Advisory Committee/Board Member, Speakers Bureau. Roche – Advisory Committee/Board Member, Speakers Bureau. Spring Bank Pharmaceuticals – Advisory Committee/Board Member, Speakers Bureau.
Heiner Wedemeyer: Abbott Laboratories – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Abbott Molecular – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Albireo Pharma – Advisory Committee/Board Member, Consultant, Speakers Bureau. AstraZeneca – Advisory Committee/Board Member, Consultant, Speakers Bureau. Atea Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speakers Bureau. BioMarin Pharmaceutical – Advisory Committee/Board Member, Consultant, Speakers Bureau. Biotest – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speakers Bureau. CSL Behring – Advisory Committee/Board Member, Consultant, Speakers Bureau. Dr. Falk Pharma – Advisory Committee/Board Member, Consultant, Speakers Bureau. Falk Foundation – Advisory Committee/Board Member, Consultant, Speakers Bureau. Gilead Sciences, Inc. – Advisory Committee/Board Member, Consultant, Speakers Bureau. GSK – Advisory Committee/Board Member, Consultant, Speakers Bureau. Janssen – Advisory Committee/Board Member, Consultant, Speakers Bureau. Lilly Deutschland – Advisory Committee/Board Member, Consultant, Speakers Bureau. Merck Sharp & Dohme – Advisory Committee/Board Member, Consultant, Speakers Bureau. Mirum Pharma Germany – Advisory Committee/Board Member, Consultant, Speakers Bureau. Olink – Advisory Committee/Board Member, Consultant, Speakers Bureau. Orphalan – Advisory Committee/Board Member, Consultant, Speakers Bureau. Pfizer – Advisory Committee/Board Member, Consultant, Speakers Bureau. Roche – Advisory Committee/Board Member, Consultant, Speakers Bureau. Roche Diagnostics International – Advisory Committee/Board Member, Consultant, Speakers Bureau. Roche Pharma – Advisory Committee/Board Member, Consultant, Speakers Bureau. Swedish Orphan Biovitrum – Advisory Committee/Board Member, Consultant, Speakers Bureau. Takeda Pharma Vertrieb – Advisory Committee/Board Member, Consultant, Speakers Bureau. Vir Biotechnology – Advisory Committee/Board Member, Consultant, Speakers Bureau.
Soo Aleman, MD, PhD1, Maurizia R. Brunetto, MD2, Antje Blank, MD3, Pietro Andreone, MD4, Pavel Bogomolov, MD, PhD5, Vladimir Chulanov, MD, PhD, DSc6, Nina Mamonova, MD7, Natalia Geyvandova, MD8, Viacheslav Morozov, MD, PhD9, Olga Sagalova, PhD10, Tatiana Stepanova, MD11, Amos Lichtman, MD, MPH12, Renee-Claude Mercier, PharmD12, Dmitry Manuilov, MD12, Sarah Arterburn, MS12, Julian Schulze zur Wiesch, MD13, Markus Cornberg, MD14, Stefan Zeuzem, MD15, Pietro Lampertico, MD, PhD16, Heiner Wedemeyer, MD14. P3781 - Predictors of Undetectable Hepatitis Delta Virus RNA at 48 Weeks After End of Treatment With Bulevirtide Monotherapy in the MYR 301 Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
