Monday Poster Session
Category: Liver
P3861 - Hepatic Adenomatosis Associated With Long-Term Methylphenidate Use: A Rare Presentation in the Absence of Traditional Risk Factors
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Oluwatoyin O. Duyile, MS (she/her/hers)
Anne Marion Burnett School of Medicine at TCU
Fort Worth, TX
Presenting Author(s)
Oluwatoyin O. Duyile, MS1, Themistoklis Kourkoumpetis, MD, MS2
1Anne Marion Burnett School of Medicine at TCU, Fort Worth, TX; 2Baylor All Saints, Fort Worth, TX
Introduction: Hepatocellular adenomas are rare benign liver tumors, with hepatic adenomatosis defined as more than 10 adenomas in an otherwise normal liver. These lesions are typically associated with risk factors such as oral contraceptives, anabolic steroids, obesity, or glycogen storage disorders. Methylphenidate (MPH), a first-line medication for ADHD, has not been associated with liver tumors in humans, though murine studies have shown hepatocellular adenomas and hepatoblastomas in animals exposed to high doses. We present a case of hepatic adenomatosis in a young woman with a history of long-term MPH use and no other identifiable risk factors.
Case Description/
Methods: A 29-year-old woman was referred to transplant hepatology clinic for evaluation of multiple hepatic adenomas. She initially presented to the emergency department with persistent abdominal pain and shortness of breath following snowboarding injuries. Imaging revealed a large 17 cm hemorrhagic hepatic mass, later embolized. MRI showed more than 20 hepatic lesions with arterial hyperenhancement and hepatobiliary hypoenhancement, consistent with hepatic adenomatosis. Her BMI was 20.08 kg/m². She had no history of glycogen storage disease, only two years of oral contraceptive use in high school, and no steroid exposure. Her past medical history was significant for ADHD, for which she had been prescribed MPH intermittently for 15 years, with re-initiation two years prior to presentation. Liver enzymes normalized over time. Extensive workup including viral hepatitis serologies, autoimmune markers, tumor markers, and genetic/metabolic labs was non-revealing.
Discussion: This is the first known report of hepatic adenomatosis in a human exposed to methylphenidate without other risk factors. While MPH is not considered genotoxic, rodent studies have demonstrated hepatocellular tumors attributed to non-genotoxic cellular proliferation at high doses. No prior human studies or case reports have described MPH-associated hepatic adenomatosis. Given the lack of alternative etiologies in our patient, MPH exposure may be a contributing factor. Clinicians should be aware of this possible association, especially when evaluating patients with unexplained hepatic lesions and a history of long-term MPH use.
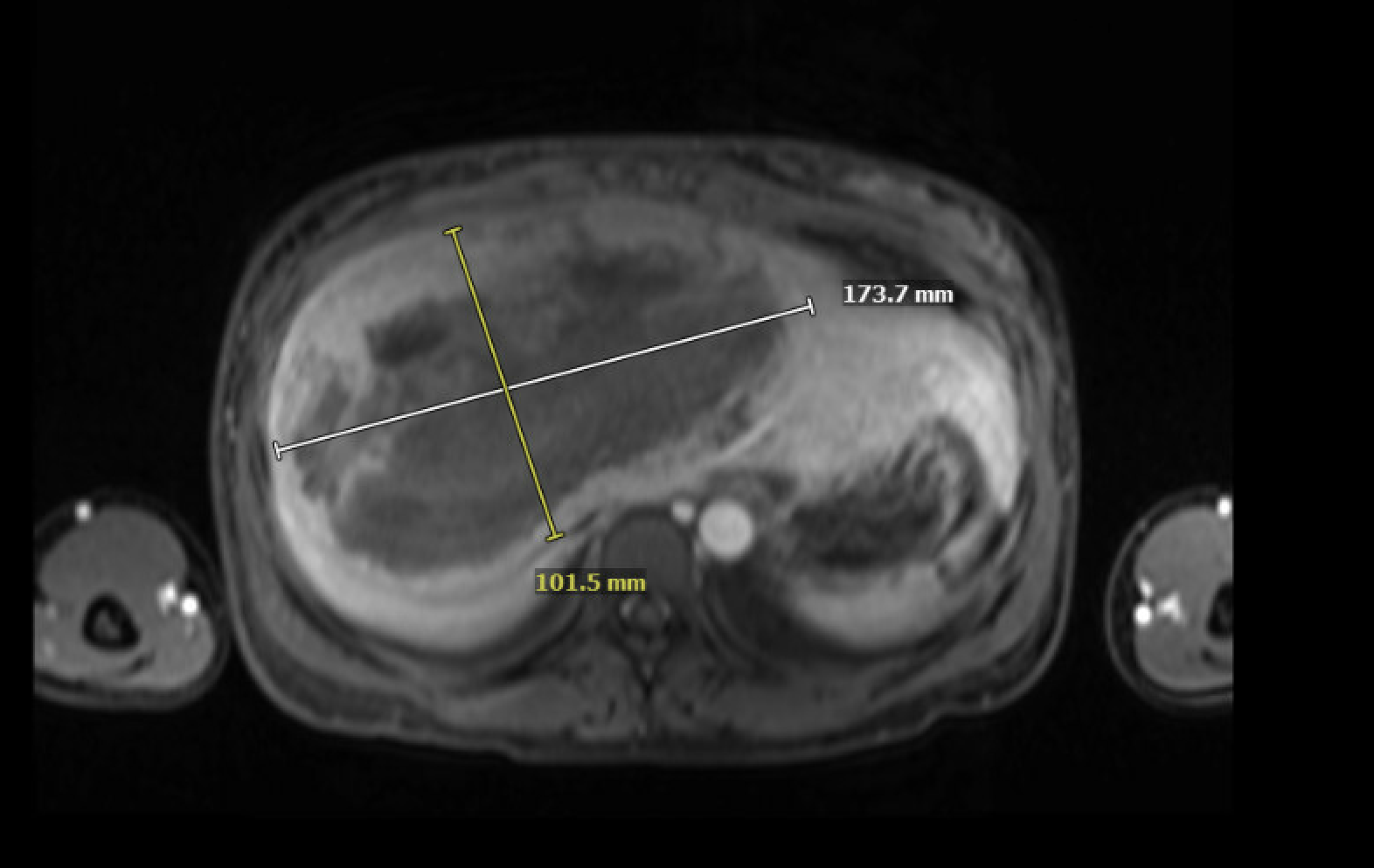
Figure: 1a. MRI imaging of the abdomen performed with and without gadolinium contrast on a 1.5 Tesla Siemens Avanto scanner. The largest mass centered in segment 4/8 measuring 17 x 10 cm (12-27) post-embolization, compatible with hemorrhage.
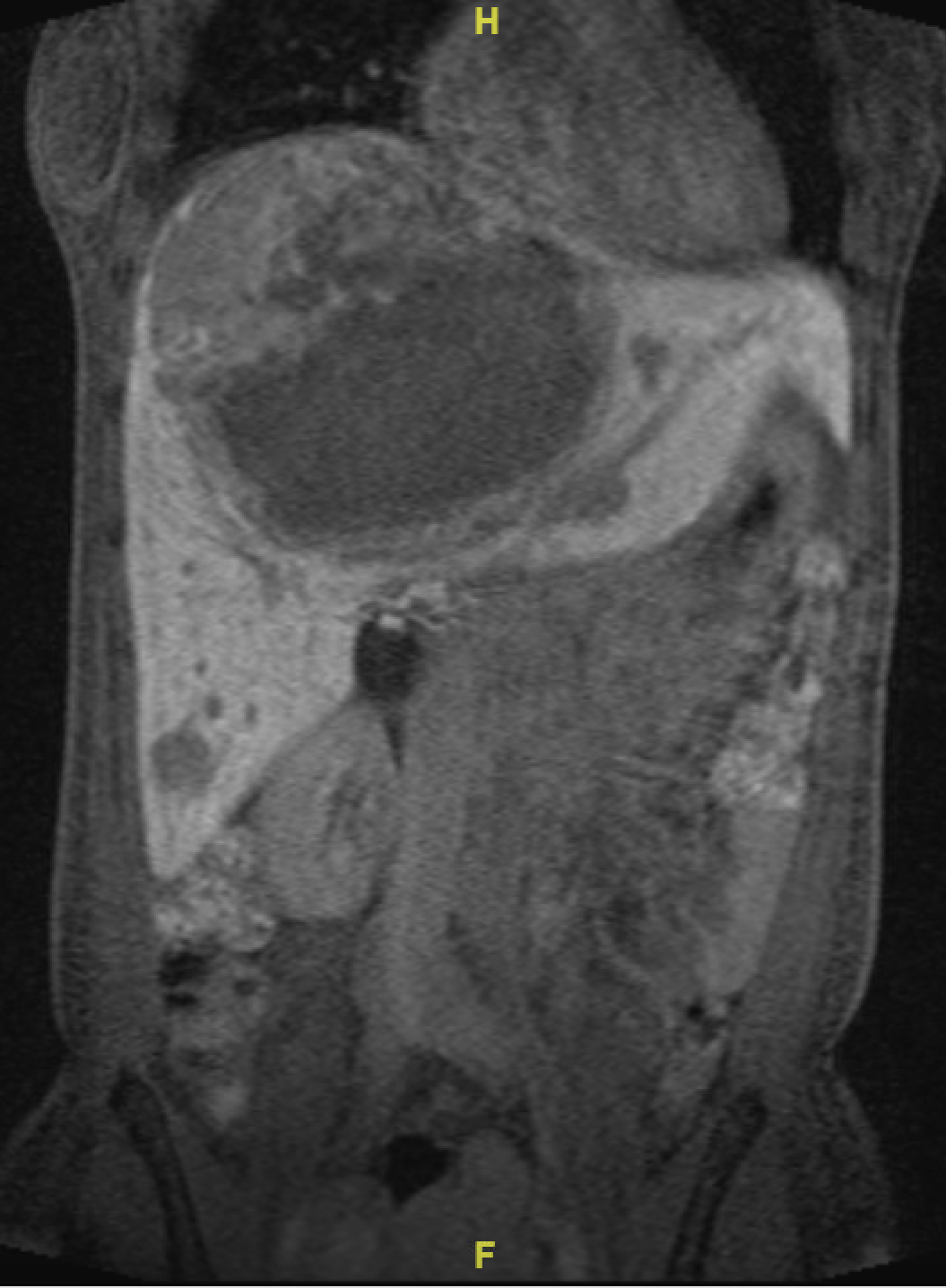
Figure: 1b. MRI imaging of the abdomen performed with and without gadolinium contrast on a 1.5 Tesla Siemens Avanto scanner. The largest mass centered in segment 4/8 measuring 17 x 10 cm (12-27) post-embolization, compatible with hemorrhage. Multiple lesions shown demonstrating hyperenhancement.
Disclosures:
Oluwatoyin Duyile indicated no relevant financial relationships.
Themistoklis Kourkoumpetis indicated no relevant financial relationships.
Oluwatoyin O. Duyile, MS1, Themistoklis Kourkoumpetis, MD, MS2. P3861 - Hepatic Adenomatosis Associated With Long-Term Methylphenidate Use: A Rare Presentation in the Absence of Traditional Risk Factors, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Anne Marion Burnett School of Medicine at TCU, Fort Worth, TX; 2Baylor All Saints, Fort Worth, TX
Introduction: Hepatocellular adenomas are rare benign liver tumors, with hepatic adenomatosis defined as more than 10 adenomas in an otherwise normal liver. These lesions are typically associated with risk factors such as oral contraceptives, anabolic steroids, obesity, or glycogen storage disorders. Methylphenidate (MPH), a first-line medication for ADHD, has not been associated with liver tumors in humans, though murine studies have shown hepatocellular adenomas and hepatoblastomas in animals exposed to high doses. We present a case of hepatic adenomatosis in a young woman with a history of long-term MPH use and no other identifiable risk factors.
Case Description/
Methods: A 29-year-old woman was referred to transplant hepatology clinic for evaluation of multiple hepatic adenomas. She initially presented to the emergency department with persistent abdominal pain and shortness of breath following snowboarding injuries. Imaging revealed a large 17 cm hemorrhagic hepatic mass, later embolized. MRI showed more than 20 hepatic lesions with arterial hyperenhancement and hepatobiliary hypoenhancement, consistent with hepatic adenomatosis. Her BMI was 20.08 kg/m². She had no history of glycogen storage disease, only two years of oral contraceptive use in high school, and no steroid exposure. Her past medical history was significant for ADHD, for which she had been prescribed MPH intermittently for 15 years, with re-initiation two years prior to presentation. Liver enzymes normalized over time. Extensive workup including viral hepatitis serologies, autoimmune markers, tumor markers, and genetic/metabolic labs was non-revealing.
Discussion: This is the first known report of hepatic adenomatosis in a human exposed to methylphenidate without other risk factors. While MPH is not considered genotoxic, rodent studies have demonstrated hepatocellular tumors attributed to non-genotoxic cellular proliferation at high doses. No prior human studies or case reports have described MPH-associated hepatic adenomatosis. Given the lack of alternative etiologies in our patient, MPH exposure may be a contributing factor. Clinicians should be aware of this possible association, especially when evaluating patients with unexplained hepatic lesions and a history of long-term MPH use.

Figure: 1a. MRI imaging of the abdomen performed with and without gadolinium contrast on a 1.5 Tesla Siemens Avanto scanner. The largest mass centered in segment 4/8 measuring 17 x 10 cm (12-27) post-embolization, compatible with hemorrhage.

Figure: 1b. MRI imaging of the abdomen performed with and without gadolinium contrast on a 1.5 Tesla Siemens Avanto scanner. The largest mass centered in segment 4/8 measuring 17 x 10 cm (12-27) post-embolization, compatible with hemorrhage. Multiple lesions shown demonstrating hyperenhancement.
Disclosures:
Oluwatoyin Duyile indicated no relevant financial relationships.
Themistoklis Kourkoumpetis indicated no relevant financial relationships.
Oluwatoyin O. Duyile, MS1, Themistoklis Kourkoumpetis, MD, MS2. P3861 - Hepatic Adenomatosis Associated With Long-Term Methylphenidate Use: A Rare Presentation in the Absence of Traditional Risk Factors, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
