Monday Poster Session
Category: Liver
P3859 - Mushroom Coffee: A Possible Risk Factor for Liver Injury
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Joshua D. Long, MD
Brown University
Providence, RI
Presenting Author(s)
Joshua D. Long, MD1, Daniel Kats, MD2, Sarah Dwyer Holland, MD, MS1, Jasneet Singh, MD1, Michael Waisberg, MD, PhD3, Kittichai Promrat, MD4
1Brown University, Providence, RI; 2Brown University / Rhode Island Hospital, Providence, RI; 3VA Providence Health Care System, Providence, RI; 4Brown University Department of Gastroenterology, Providence, RI
Introduction: Herbal-induced liver injury (HILI) must be considered in the differential diagnosis of acute hepatocellular damage or cholestasis of unknown etiology. Clinically significant HILI from Reishi (Ganoderma lucidum), a mushroom used in traditional Chinese medicine, has been rarely reported in literature. We present a case of severe liver injury after consumption of a commercially available mushroom coffee (MC) containing multiple mushrooms, including Reishi.
Case Description/
Methods: A 63-year-old male with a BMI of 33 kg/m2, hypertension, and weekly consumption of 2 glasses of wine presented with dark urine and pale stools. He began drinking 1-2 cups of MC daily in the preceding month and recently initiated atorvastatin 10 mg daily, which he took as prescribed for 4 doses. On admission, atorvastatin and MC were discontinued. His liver panel showed elevated aminotransferases consistent with hepatocellular injury (R-factor >5). Abdominal ultrasound showed echogenic liver and mild hepatomegaly. CT of the abdomen and pelvis showed peripancreatic edema, prominent portacaval and porta hepatis lymph nodes and a partially contracted gallbladder. He was discharged 4 days later.
Eight days after admission, despite continued cessation of atorvastatin and MC, his labs were still significantly elevated, and he was readmitted. Liver biopsy demonstrated interface and lobular activity with abundant plasma cells and neutrophils, suggestive of autoimmune hepatitis with superimposed DILI. He was discharged after a 3-day hospital stay. Later that week, he reported worsening fatigue and started a 40 mg prednisone taper, and subsequently, azathioprine 50 mg daily. He eventually stopped all immunosuppression given concerns regarding side effects and was monitored with monthly labs. His liver enzymes have remained normal for nearly 5 months after cessation of immunosuppressive therapy.
Discussion: When evaluating the etiology of new-onset liver injury without obvious cause, obtaining a thorough history, including recently initiated over-the-counter products or dietary supplements is crucial. In our case, MC or atorvastatin alone, or alternatively, the combination of MC, atorvastatin, and alcohol consumption may have caused the patient's liver injury. Regardless, prompt identification and cessation of all the above hepatotoxic agents, along with corticosteroid treatment, allowed this patient to achieve clinical recovery.
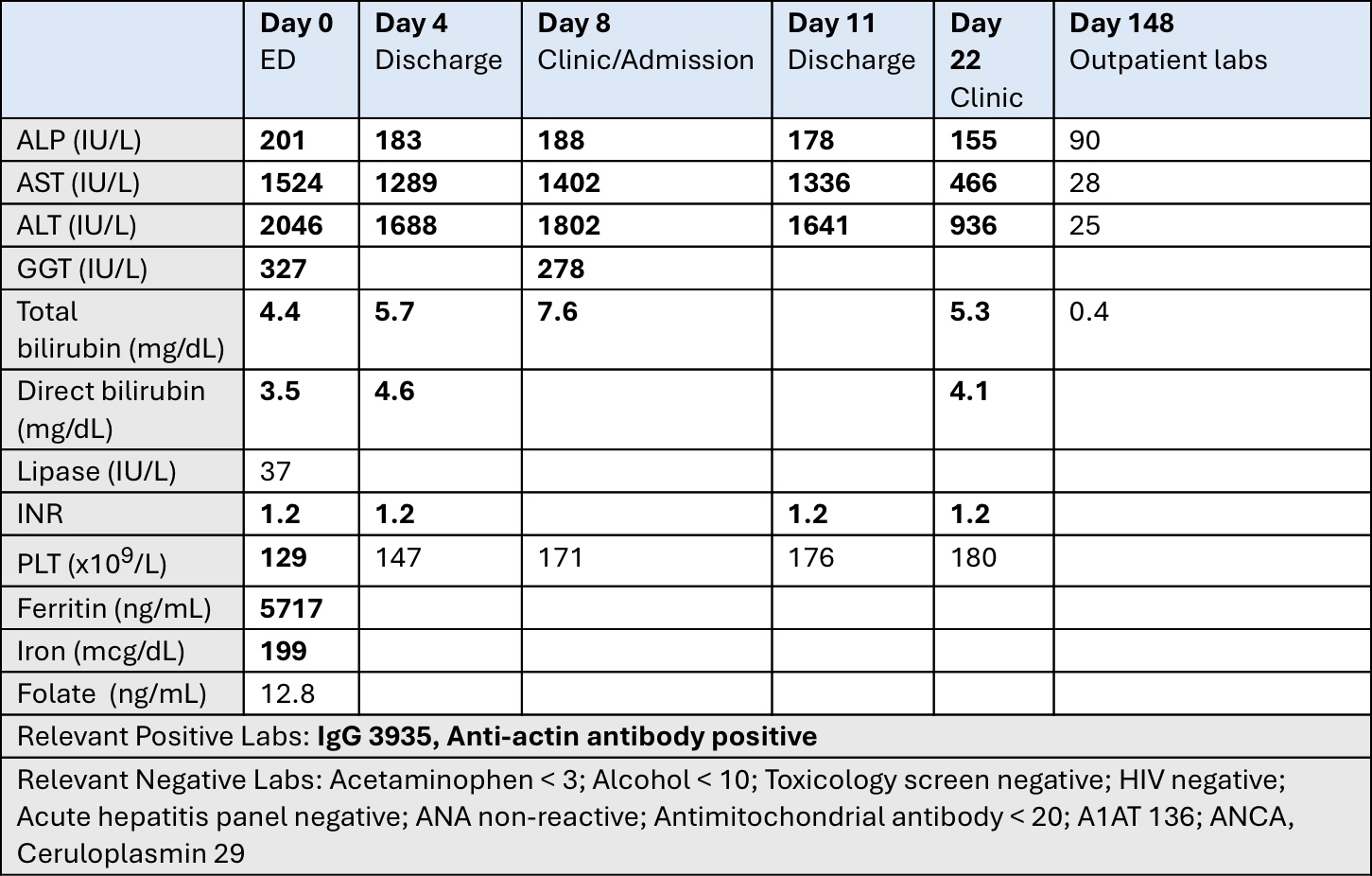
Figure: Table 1. Lab values. ALP: alkaline phosphatase; AST: aspartate aminotransferase; ALT: alanine transaminase; GGT: gamma-glutamyl transferase; INR: international normalized ratio; PLT: platelets; A1AT: alpha-1 antitrypsin.

Figure: Figure 1. H&E-stained liver tissue showing interface and lobular activity with abundant plasma cells and neutrophils, suggestive of autoimmune hepatitis with superimposed DILI.
Disclosures:
Joshua D. Long indicated no relevant financial relationships.
Daniel Kats indicated no relevant financial relationships.
Sarah Dwyer Holland indicated no relevant financial relationships.
Jasneet Singh indicated no relevant financial relationships.
Michael Waisberg indicated no relevant financial relationships.
Kittichai Promrat indicated no relevant financial relationships.
Joshua D. Long, MD1, Daniel Kats, MD2, Sarah Dwyer Holland, MD, MS1, Jasneet Singh, MD1, Michael Waisberg, MD, PhD3, Kittichai Promrat, MD4. P3859 - Mushroom Coffee: A Possible Risk Factor for Liver Injury, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Brown University, Providence, RI; 2Brown University / Rhode Island Hospital, Providence, RI; 3VA Providence Health Care System, Providence, RI; 4Brown University Department of Gastroenterology, Providence, RI
Introduction: Herbal-induced liver injury (HILI) must be considered in the differential diagnosis of acute hepatocellular damage or cholestasis of unknown etiology. Clinically significant HILI from Reishi (Ganoderma lucidum), a mushroom used in traditional Chinese medicine, has been rarely reported in literature. We present a case of severe liver injury after consumption of a commercially available mushroom coffee (MC) containing multiple mushrooms, including Reishi.
Case Description/
Methods: A 63-year-old male with a BMI of 33 kg/m2, hypertension, and weekly consumption of 2 glasses of wine presented with dark urine and pale stools. He began drinking 1-2 cups of MC daily in the preceding month and recently initiated atorvastatin 10 mg daily, which he took as prescribed for 4 doses. On admission, atorvastatin and MC were discontinued. His liver panel showed elevated aminotransferases consistent with hepatocellular injury (R-factor >5). Abdominal ultrasound showed echogenic liver and mild hepatomegaly. CT of the abdomen and pelvis showed peripancreatic edema, prominent portacaval and porta hepatis lymph nodes and a partially contracted gallbladder. He was discharged 4 days later.
Eight days after admission, despite continued cessation of atorvastatin and MC, his labs were still significantly elevated, and he was readmitted. Liver biopsy demonstrated interface and lobular activity with abundant plasma cells and neutrophils, suggestive of autoimmune hepatitis with superimposed DILI. He was discharged after a 3-day hospital stay. Later that week, he reported worsening fatigue and started a 40 mg prednisone taper, and subsequently, azathioprine 50 mg daily. He eventually stopped all immunosuppression given concerns regarding side effects and was monitored with monthly labs. His liver enzymes have remained normal for nearly 5 months after cessation of immunosuppressive therapy.
Discussion: When evaluating the etiology of new-onset liver injury without obvious cause, obtaining a thorough history, including recently initiated over-the-counter products or dietary supplements is crucial. In our case, MC or atorvastatin alone, or alternatively, the combination of MC, atorvastatin, and alcohol consumption may have caused the patient's liver injury. Regardless, prompt identification and cessation of all the above hepatotoxic agents, along with corticosteroid treatment, allowed this patient to achieve clinical recovery.

Figure: Table 1. Lab values. ALP: alkaline phosphatase; AST: aspartate aminotransferase; ALT: alanine transaminase; GGT: gamma-glutamyl transferase; INR: international normalized ratio; PLT: platelets; A1AT: alpha-1 antitrypsin.

Figure: Figure 1. H&E-stained liver tissue showing interface and lobular activity with abundant plasma cells and neutrophils, suggestive of autoimmune hepatitis with superimposed DILI.
Disclosures:
Joshua D. Long indicated no relevant financial relationships.
Daniel Kats indicated no relevant financial relationships.
Sarah Dwyer Holland indicated no relevant financial relationships.
Jasneet Singh indicated no relevant financial relationships.
Michael Waisberg indicated no relevant financial relationships.
Kittichai Promrat indicated no relevant financial relationships.
Joshua D. Long, MD1, Daniel Kats, MD2, Sarah Dwyer Holland, MD, MS1, Jasneet Singh, MD1, Michael Waisberg, MD, PhD3, Kittichai Promrat, MD4. P3859 - Mushroom Coffee: A Possible Risk Factor for Liver Injury, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
