Monday Poster Session
Category: Liver
P3950 - When Is a Liver Biopsy Warranted? A Case of Immune Checkpoint Inhibitor Hepatitis With Diagnostic Uncertainty
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- CN
Cristina Natha, MD
McGovern Medical School at UTHealth
Houston, TX
Presenting Author(s)
Cristina Natha, MD1, Varun Vemulapalli, MD1, Mariam Rizvi, MD2, George Ishac, MD2, Aastha Bharwad, MD2, Akshata Moghe, MD3
1McGovern Medical School at UTHealth, Houston, TX; 2University of Texas at Houston, Houston, TX; 3University of Texas Health, McGovern Medical School, Houston, TX
Introduction: Immune checkpoint inhibitors (ICIs) have become a cornerstone of cancer therapy across a range of malignancies. However, they are increasingly associated with immune-related adverse events including hepatotoxicity. While ICI-induced hepatitis is frequently diagnosed based on clinical and laboratory findings, the role of liver biopsy remains important in cases complicated by recent exposure to other potentially hepatotoxic agents. As ICI use expands, diagnostic uncertainty in liver injury is increasingly common. However, clear criteria for when to pursue liver biopsy are lacking. Determining when to biopsy is critical, as premature or unnecessary immunosuppression may obscure alternative diagnoses or disrupt effective oncologic treatment.
Case Description/
Methods: A 68-year-old male with metastatic renal cell carcinoma (RCC) on pembrolizumab (ICI) and lenvatinib (kinase inhibitor) presented after routine oncology labs revealed a significant new elevation in liver enzymes including AST 3,885 U/L and ALT >3,300 U/L, total bilirubin 7.1 mg/dL, and INR 2.13. He was asymptomatic aside from scleral icterus. Notably, he had recently completed a 7-day course of cephalexin for an arm wound infection, also raising concern for drug-induced liver injury (DILI) from antibiotics. Due to diagnostic uncertainty, both cancer therapies were held. Abdominal imaging showed hepatic cysts without biliary obstruction or metastases. Comprehensive evaluation for infectious, autoimmune, and metabolic liver disease was unrevealing. A percutaneous liver biopsy was performed and histopathology revealed zone 3 confluent and bridging necrosis with parenchymal collapse and lobular and portal inflammation—findings consistent with ICI-induced hepatitis. High-dose corticosteroids were initiated, with rapid improvement of liver enzymes.
Discussion: This case illustrates the diagnostic utility of a liver biopsy in evaluating acute liver injury in patients receiving ICIs, especially when other hepatotoxic exposures confound the clinical picture. In this case, ICI use was critical for treatment of RCC, and confirmation of the cause of hepatitis was essential to avoid unnecessary discontinuation of ICI therapy. Histologic confirmation excluded alternative causes and enabled more informed decisions regarding future cancer treatment including risk stratification for ICI rechallenge. As ICIs are increasingly used in complex clinical settings, biopsy should be considered when diagnostic uncertainty may affect management.
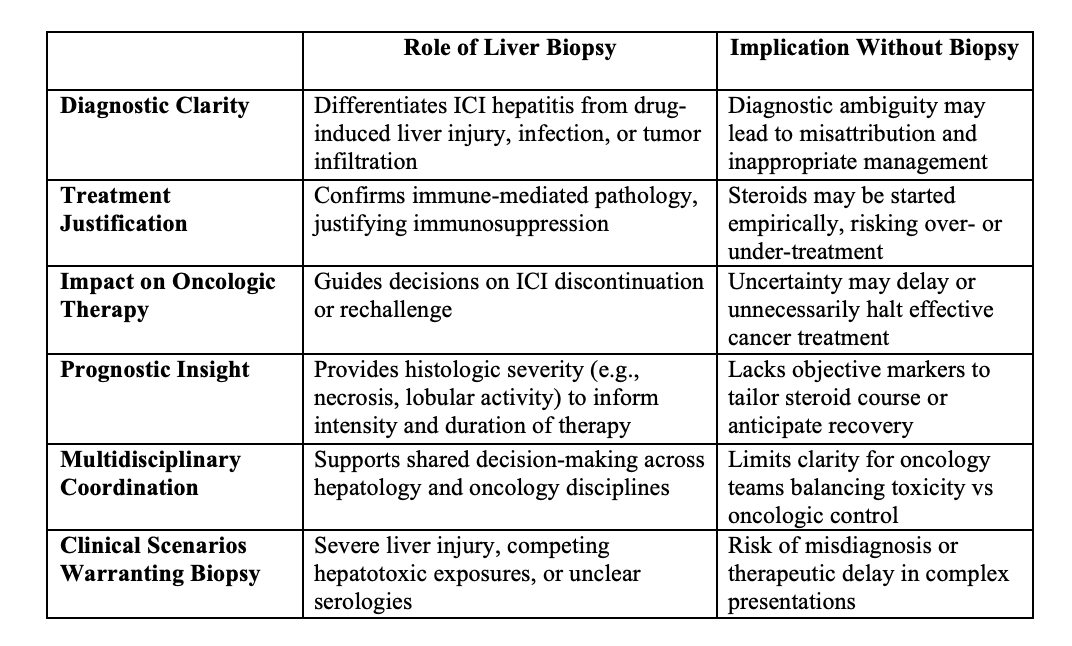
Figure: Diagnostic and Therapeutic Implications of Liver Biopsy in Suspected ICI-Induced Hepatitis
Disclosures:
Cristina Natha indicated no relevant financial relationships.
Varun Vemulapalli indicated no relevant financial relationships.
Mariam Rizvi indicated no relevant financial relationships.
George Ishac indicated no relevant financial relationships.
Aastha Bharwad indicated no relevant financial relationships.
Akshata Moghe indicated no relevant financial relationships.
Cristina Natha, MD1, Varun Vemulapalli, MD1, Mariam Rizvi, MD2, George Ishac, MD2, Aastha Bharwad, MD2, Akshata Moghe, MD3. P3950 - When Is a Liver Biopsy Warranted? A Case of Immune Checkpoint Inhibitor Hepatitis With Diagnostic Uncertainty, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1McGovern Medical School at UTHealth, Houston, TX; 2University of Texas at Houston, Houston, TX; 3University of Texas Health, McGovern Medical School, Houston, TX
Introduction: Immune checkpoint inhibitors (ICIs) have become a cornerstone of cancer therapy across a range of malignancies. However, they are increasingly associated with immune-related adverse events including hepatotoxicity. While ICI-induced hepatitis is frequently diagnosed based on clinical and laboratory findings, the role of liver biopsy remains important in cases complicated by recent exposure to other potentially hepatotoxic agents. As ICI use expands, diagnostic uncertainty in liver injury is increasingly common. However, clear criteria for when to pursue liver biopsy are lacking. Determining when to biopsy is critical, as premature or unnecessary immunosuppression may obscure alternative diagnoses or disrupt effective oncologic treatment.
Case Description/
Methods: A 68-year-old male with metastatic renal cell carcinoma (RCC) on pembrolizumab (ICI) and lenvatinib (kinase inhibitor) presented after routine oncology labs revealed a significant new elevation in liver enzymes including AST 3,885 U/L and ALT >3,300 U/L, total bilirubin 7.1 mg/dL, and INR 2.13. He was asymptomatic aside from scleral icterus. Notably, he had recently completed a 7-day course of cephalexin for an arm wound infection, also raising concern for drug-induced liver injury (DILI) from antibiotics. Due to diagnostic uncertainty, both cancer therapies were held. Abdominal imaging showed hepatic cysts without biliary obstruction or metastases. Comprehensive evaluation for infectious, autoimmune, and metabolic liver disease was unrevealing. A percutaneous liver biopsy was performed and histopathology revealed zone 3 confluent and bridging necrosis with parenchymal collapse and lobular and portal inflammation—findings consistent with ICI-induced hepatitis. High-dose corticosteroids were initiated, with rapid improvement of liver enzymes.
Discussion: This case illustrates the diagnostic utility of a liver biopsy in evaluating acute liver injury in patients receiving ICIs, especially when other hepatotoxic exposures confound the clinical picture. In this case, ICI use was critical for treatment of RCC, and confirmation of the cause of hepatitis was essential to avoid unnecessary discontinuation of ICI therapy. Histologic confirmation excluded alternative causes and enabled more informed decisions regarding future cancer treatment including risk stratification for ICI rechallenge. As ICIs are increasingly used in complex clinical settings, biopsy should be considered when diagnostic uncertainty may affect management.

Figure: Diagnostic and Therapeutic Implications of Liver Biopsy in Suspected ICI-Induced Hepatitis
Disclosures:
Cristina Natha indicated no relevant financial relationships.
Varun Vemulapalli indicated no relevant financial relationships.
Mariam Rizvi indicated no relevant financial relationships.
George Ishac indicated no relevant financial relationships.
Aastha Bharwad indicated no relevant financial relationships.
Akshata Moghe indicated no relevant financial relationships.
Cristina Natha, MD1, Varun Vemulapalli, MD1, Mariam Rizvi, MD2, George Ishac, MD2, Aastha Bharwad, MD2, Akshata Moghe, MD3. P3950 - When Is a Liver Biopsy Warranted? A Case of Immune Checkpoint Inhibitor Hepatitis With Diagnostic Uncertainty, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
