Monday Poster Session
Category: Small Intestine
P4022 - Drug-Induced Intestinal Necrosis: Disproportionality Analysis Using the FDA Adverse Event Reporting System (FAERS) Database
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Rohan Karkra, MBBS
Rutgers New Jersey Medical School
Newark, NJ
Presenting Author(s)
Rohan Karkra, MBBS, Michael Bebawy, DO, Steven Rella, DO, Dean Magat, MD, Kaveh Hajifathalian, MD, Ahmed Al-Khazraji, MD
Rutgers New Jersey Medical School, Newark, NJ
Introduction: Drug-induced intestinal necrosis is a severe and potentially life-threatening condition characterized by the death of the intestinal tissue. Typical symptoms include abdominal pain, vomiting, diarrhea, and fever. This can rapidly progress to severe complications such as septic shock and peritonitis. Early recognition and prompt discontinuation of the offending drug are crucial to mitigate the risk of severe complications and improve patient outcomes. This is a real world pharmacovigilance study evaluating the association between various drugs and intestinal necrosis.
Methods: The Food and Drug Administration (FDA) maintains the Adverse Event Reporting System (FAERS) database. As of December 31, 2024, the database contained 30,179,725 reported events. This database was queried to identify all reported cases of ‘Gastrointestinal necrosis’, ‘Gastrointestinal mucosal necrosis’, ‘intestinal gangrene’, and ‘gastrointestinal gangrene’. All medications implicated were identified by their generic names. The database does not report how the diagnosis of necrosis was confirmed, nor whether other causes were ruled out. A disproportionality analysis was conducted, wherein Reporting Odds Ratios (ROR) were calculated. An ROR >1 is considered a significant signal.
Results: A total of 3752 cases were reported to the FAERS from 1970-2024. 3701 were characterized as severe, including 1589 deaths (42.35%). The most common reported drugs were – Docetaxel (3.86%; ROR: 13.9 [11.77, 16.41]; p< 0.05), Adalimumab (3.46%; ROR: 1.45 [1.22, 1.73]; p< 0.05), Fluorouracil (3.38%; ROR: 13.89 [11.64, 16.58]; p< 0.05), Clozapine (3.22%; ROR: 6.99 [5.83, 8.38]; p< 0.05), Cyclophosphamide (3.14%; ROR: 6.94 [5.83, 8.26]; p< 0.05), Methotrexate (3.11%; ROR: 5.00 [4.16, 6.01]; p< 0.05), Paclitaxel (3.04%; ROR: 9.09 [7.54, 10.95]; p< 0.05), Bevacizumab (2.77%; ROR: 7.95 [6.54, 9.66]; p< 0.05), Cytarabine (2.77%; ROR: 15.43 [12.70, 18.76]; p< 0.05), and Cisplatin (2.48%; ROR: 10.84 [8.82, 13.32]; p< 0.05).
Discussion: Docetaxel was the most reported cause of drug-induced intestinal necrosis (3.6% of all reports), while Cytarabine had the highest ROR (15.43) amongst the most frequently reported drugs. A high degree of suspicion should be employed when patients present with warning symptoms after a recent change in medications. Such patients should discontinue these medications when possible, and immediate supportive care should be instituted.
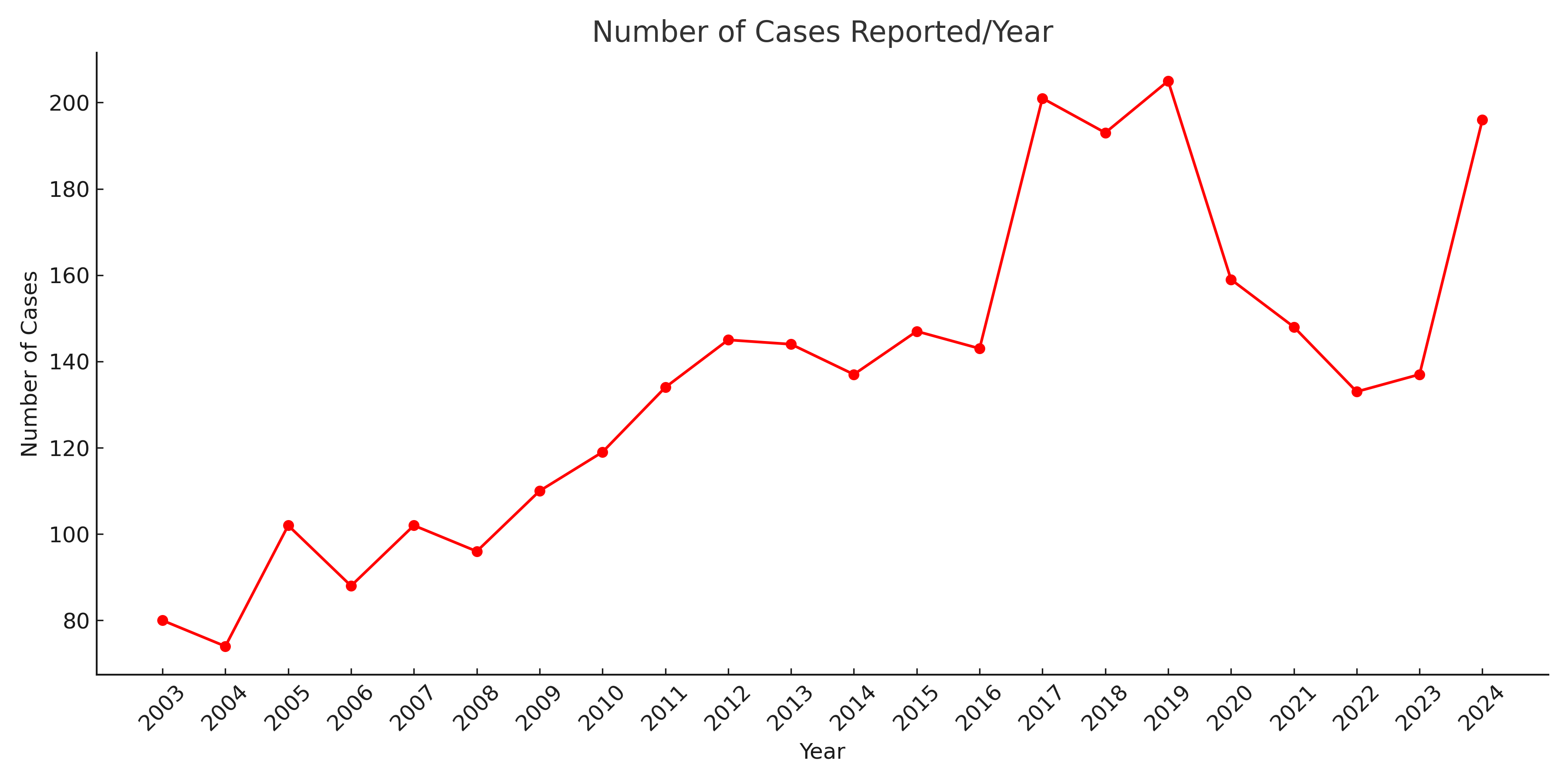
Figure: Number of cases of drug induced intestinal necrosis reported per year
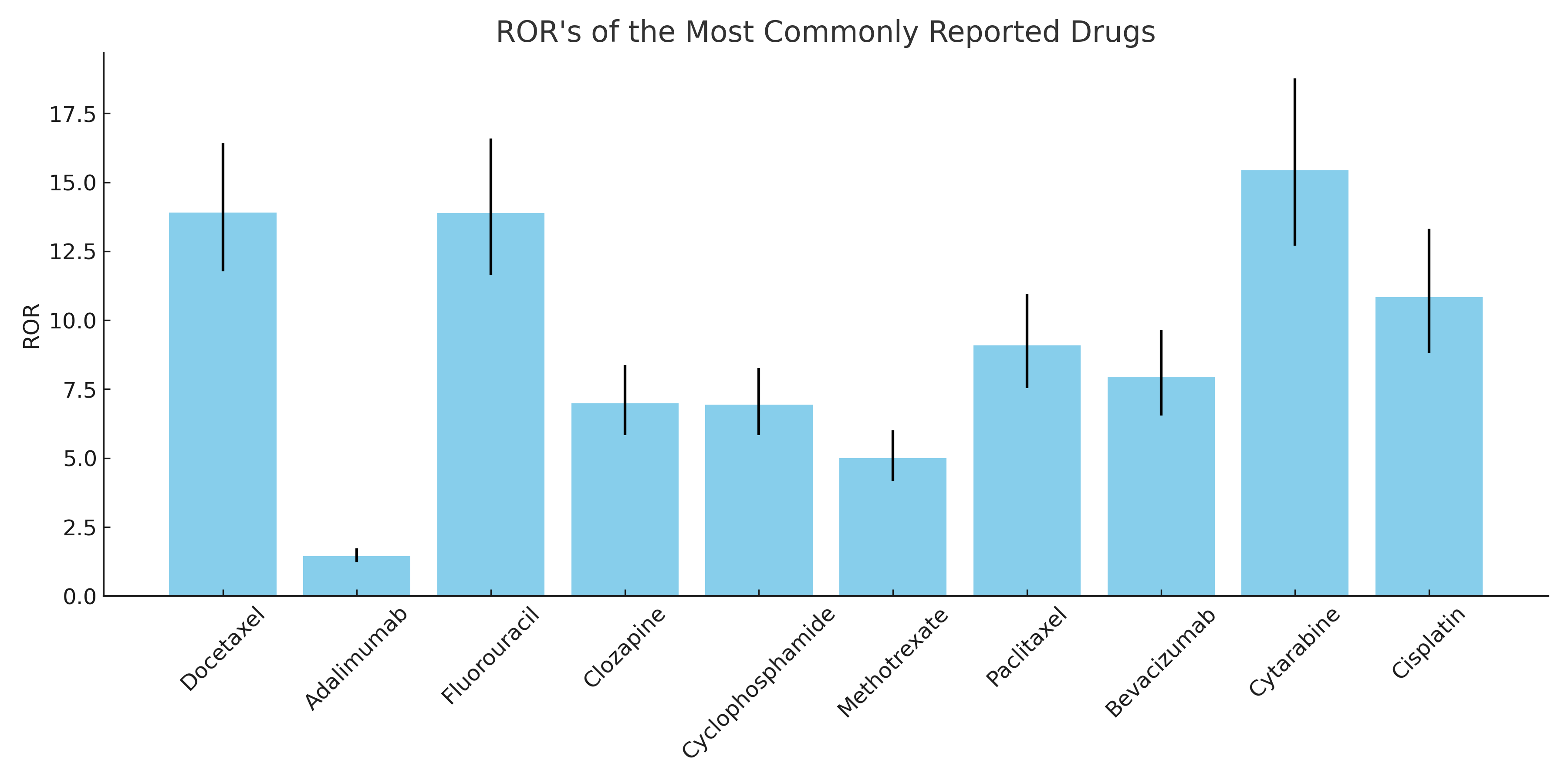
Figure: Reporting Odds Ratio (ROR) for the most commonly reported drugs
Disclosures:
Rohan Karkra indicated no relevant financial relationships.
Michael Bebawy indicated no relevant financial relationships.
Steven Rella indicated no relevant financial relationships.
Dean Magat indicated no relevant financial relationships.
Kaveh Hajifathalian indicated no relevant financial relationships.
Ahmed Al-Khazraji indicated no relevant financial relationships.
Rohan Karkra, MBBS, Michael Bebawy, DO, Steven Rella, DO, Dean Magat, MD, Kaveh Hajifathalian, MD, Ahmed Al-Khazraji, MD. P4022 - Drug-Induced Intestinal Necrosis: Disproportionality Analysis Using the FDA Adverse Event Reporting System (FAERS) Database, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Rutgers New Jersey Medical School, Newark, NJ
Introduction: Drug-induced intestinal necrosis is a severe and potentially life-threatening condition characterized by the death of the intestinal tissue. Typical symptoms include abdominal pain, vomiting, diarrhea, and fever. This can rapidly progress to severe complications such as septic shock and peritonitis. Early recognition and prompt discontinuation of the offending drug are crucial to mitigate the risk of severe complications and improve patient outcomes. This is a real world pharmacovigilance study evaluating the association between various drugs and intestinal necrosis.
Methods: The Food and Drug Administration (FDA) maintains the Adverse Event Reporting System (FAERS) database. As of December 31, 2024, the database contained 30,179,725 reported events. This database was queried to identify all reported cases of ‘Gastrointestinal necrosis’, ‘Gastrointestinal mucosal necrosis’, ‘intestinal gangrene’, and ‘gastrointestinal gangrene’. All medications implicated were identified by their generic names. The database does not report how the diagnosis of necrosis was confirmed, nor whether other causes were ruled out. A disproportionality analysis was conducted, wherein Reporting Odds Ratios (ROR) were calculated. An ROR >1 is considered a significant signal.
Results: A total of 3752 cases were reported to the FAERS from 1970-2024. 3701 were characterized as severe, including 1589 deaths (42.35%). The most common reported drugs were – Docetaxel (3.86%; ROR: 13.9 [11.77, 16.41]; p< 0.05), Adalimumab (3.46%; ROR: 1.45 [1.22, 1.73]; p< 0.05), Fluorouracil (3.38%; ROR: 13.89 [11.64, 16.58]; p< 0.05), Clozapine (3.22%; ROR: 6.99 [5.83, 8.38]; p< 0.05), Cyclophosphamide (3.14%; ROR: 6.94 [5.83, 8.26]; p< 0.05), Methotrexate (3.11%; ROR: 5.00 [4.16, 6.01]; p< 0.05), Paclitaxel (3.04%; ROR: 9.09 [7.54, 10.95]; p< 0.05), Bevacizumab (2.77%; ROR: 7.95 [6.54, 9.66]; p< 0.05), Cytarabine (2.77%; ROR: 15.43 [12.70, 18.76]; p< 0.05), and Cisplatin (2.48%; ROR: 10.84 [8.82, 13.32]; p< 0.05).
Discussion: Docetaxel was the most reported cause of drug-induced intestinal necrosis (3.6% of all reports), while Cytarabine had the highest ROR (15.43) amongst the most frequently reported drugs. A high degree of suspicion should be employed when patients present with warning symptoms after a recent change in medications. Such patients should discontinue these medications when possible, and immediate supportive care should be instituted.

Figure: Number of cases of drug induced intestinal necrosis reported per year

Figure: Reporting Odds Ratio (ROR) for the most commonly reported drugs
Disclosures:
Rohan Karkra indicated no relevant financial relationships.
Michael Bebawy indicated no relevant financial relationships.
Steven Rella indicated no relevant financial relationships.
Dean Magat indicated no relevant financial relationships.
Kaveh Hajifathalian indicated no relevant financial relationships.
Ahmed Al-Khazraji indicated no relevant financial relationships.
Rohan Karkra, MBBS, Michael Bebawy, DO, Steven Rella, DO, Dean Magat, MD, Kaveh Hajifathalian, MD, Ahmed Al-Khazraji, MD. P4022 - Drug-Induced Intestinal Necrosis: Disproportionality Analysis Using the FDA Adverse Event Reporting System (FAERS) Database, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

