Monday Poster Session
Category: Stomach and Spleen
P4189 - Beyond Erectile Dysfunction: Investigating the Protective Effect of PDE-5 Inhibitors Against Gastric Cancer
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Chisom Nwaneki, MD, MPH
Saint Peter's University Hospital, New Brunswick, NJ
New Brunswick, NJ
Presenting Author(s)
Chisom Nwaneki, MD, MPH1, Maurice Facey, MD2, Jaime A. Perez, PhD3, Juanita L.. Merchant, MD, PhD4, Fabio Cominelli, MD, PhD3, Bianca Islam, MD, PhD3
1Saint Peter's University Hospital, New Brunswick, NJ, New Brunswick, NJ; 2University Hospitals Cleveland Medical Center, Cleveland, OH; 3University Hospitals Cleveland Medical Center, Case Western Reserve University, Cleveland, OH; 4University of Arizona Cancer Center, Tucson, AZ
Introduction: Aside from its use in the treatment of erectile dysfunction, there is growing evidence to suggest anti-cancer properties of PDE-5 inhibitors (PDE5i). Given the anti-tumor effects described in both animal and human studies for colon cancer, we hypothesize that other GI cancers, like gastric cancer, may respond to the anti-tumor effects of PDE5i, Therefore, we explored the incidence of gastric cancer among patients chronically taking PDE5i compared to the general population (not taking PDE5i).
Methods: We conducted a retrospective cohort study using TriNetX, a multi-institutional database in the United States. We included adult males aged 60 and above who have been taking Sildenafil, Tadalafil, and Vardenafil from January 1, 2015, to April 30, 2025, and a matched cohort not taking PDE5i. Means, proportions, and percentages were used to describe the demographic features of the study population. The primary outcome of interest is a diagnosis of gastric cancer or colon cancer in the study population, described using incidence rates and odds ratios, after adjusting for potential cofounders.
Results: There were 795,289 patients taking PDE5i and 9,513,128 not taking PDE5i before matching. After matching, there were 794,925 patients in both cohorts. Patients on PDE5i were found to have a statistically significant reduction in rates of gastric cancer (OR 0.7, 95%CI 0.65-0.76, p < 0.001). Additionally, there was a statistically significant reduction in rates of colon cancer in the PDE5i group (OR 0.68, 95% CI 0.65-0.71, p< 0.001) compared to the non-PDE5i control. Upon stratification based on H. pylori status, patients with H. pylori on PDE-5i remained at an increased risk of gastric cancer (OR 2.33, 1.11-4.89, p= 0.0218).
Discussion: Our study utilized a comprehensive health network (TriNetX) that evaluated patients on PDE-5 inhibitors (Sildenafil, Tadalafil, and Vardenafil) and found that the rates of developing gastric cancer and colon cancer were lower than those not on these therapies. Additionally, our study highlighted those patients with a prior or current H. pylori infection, despite being on PDE5i, still had an increased risk of gastric cancer. Future prospective studies are needed to further explore the anti-tumor effects of PDE-5 inhibitors on gastric cancer.
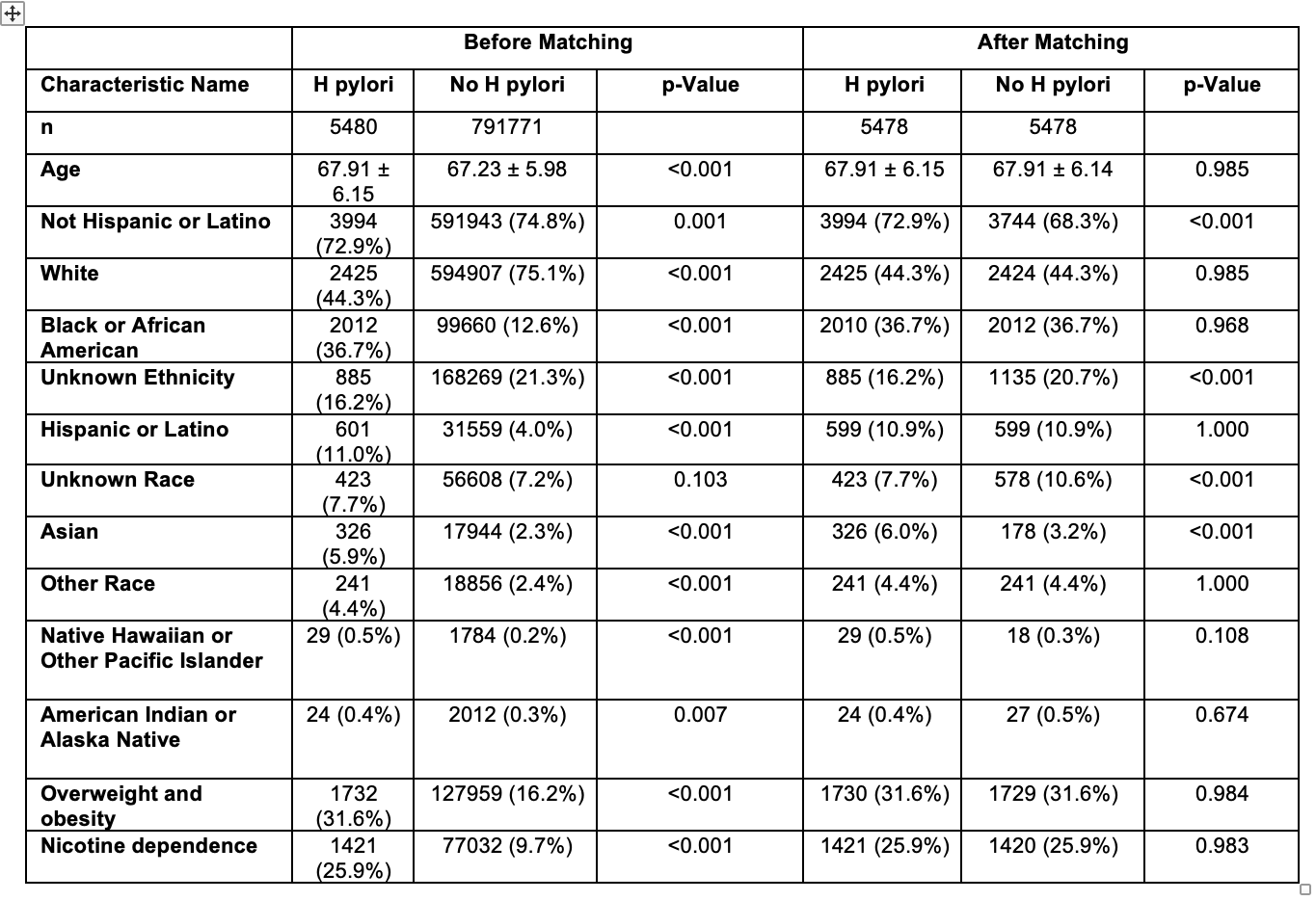
Figure: Table 1. Baseline characteristics of patients before and after propensity score matching, including p-values.
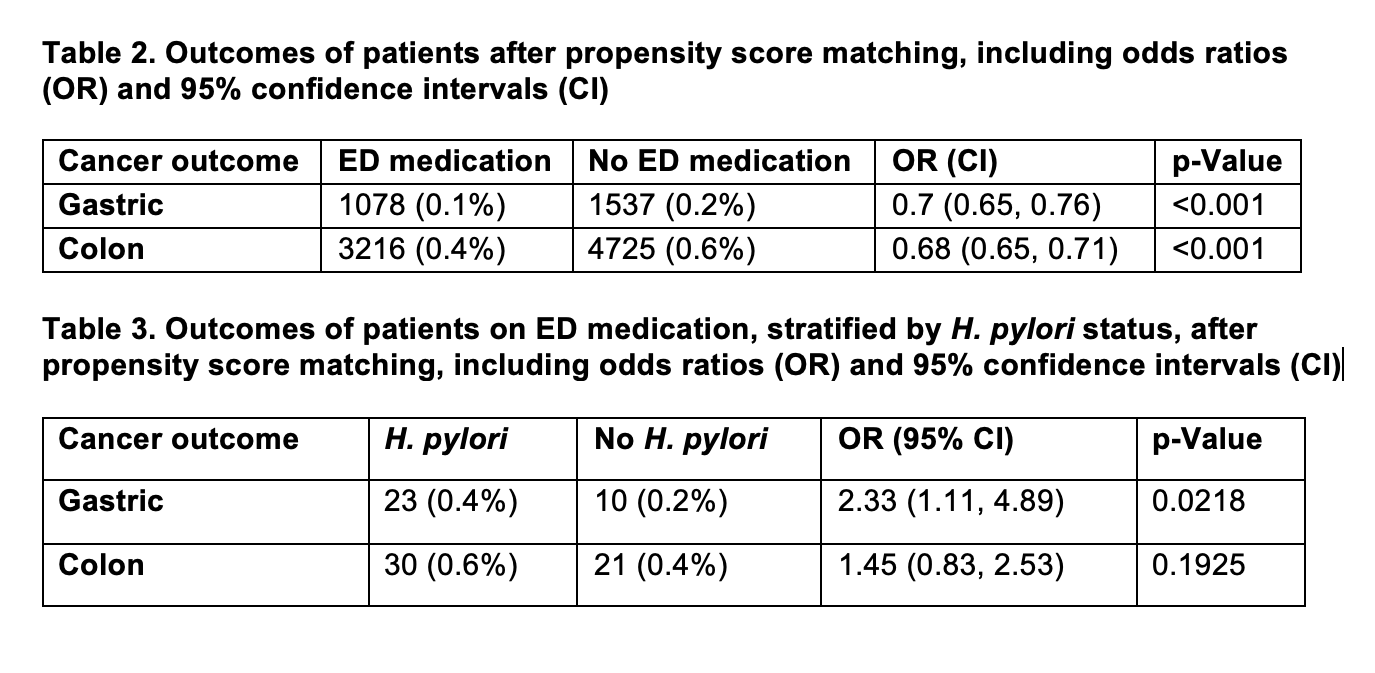
Figure: Tables 2 and 3. Outcomes with adjusted OR
Disclosures:
Chisom Nwaneki indicated no relevant financial relationships.
Maurice Facey indicated no relevant financial relationships.
Jaime Perez indicated no relevant financial relationships.
Juanita Merchant indicated no relevant financial relationships.
Fabio Cominelli indicated no relevant financial relationships.
Bianca Islam indicated no relevant financial relationships.
Chisom Nwaneki, MD, MPH1, Maurice Facey, MD2, Jaime A. Perez, PhD3, Juanita L.. Merchant, MD, PhD4, Fabio Cominelli, MD, PhD3, Bianca Islam, MD, PhD3. P4189 - Beyond Erectile Dysfunction: Investigating the Protective Effect of PDE-5 Inhibitors Against Gastric Cancer, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Saint Peter's University Hospital, New Brunswick, NJ, New Brunswick, NJ; 2University Hospitals Cleveland Medical Center, Cleveland, OH; 3University Hospitals Cleveland Medical Center, Case Western Reserve University, Cleveland, OH; 4University of Arizona Cancer Center, Tucson, AZ
Introduction: Aside from its use in the treatment of erectile dysfunction, there is growing evidence to suggest anti-cancer properties of PDE-5 inhibitors (PDE5i). Given the anti-tumor effects described in both animal and human studies for colon cancer, we hypothesize that other GI cancers, like gastric cancer, may respond to the anti-tumor effects of PDE5i, Therefore, we explored the incidence of gastric cancer among patients chronically taking PDE5i compared to the general population (not taking PDE5i).
Methods: We conducted a retrospective cohort study using TriNetX, a multi-institutional database in the United States. We included adult males aged 60 and above who have been taking Sildenafil, Tadalafil, and Vardenafil from January 1, 2015, to April 30, 2025, and a matched cohort not taking PDE5i. Means, proportions, and percentages were used to describe the demographic features of the study population. The primary outcome of interest is a diagnosis of gastric cancer or colon cancer in the study population, described using incidence rates and odds ratios, after adjusting for potential cofounders.
Results: There were 795,289 patients taking PDE5i and 9,513,128 not taking PDE5i before matching. After matching, there were 794,925 patients in both cohorts. Patients on PDE5i were found to have a statistically significant reduction in rates of gastric cancer (OR 0.7, 95%CI 0.65-0.76, p < 0.001). Additionally, there was a statistically significant reduction in rates of colon cancer in the PDE5i group (OR 0.68, 95% CI 0.65-0.71, p< 0.001) compared to the non-PDE5i control. Upon stratification based on H. pylori status, patients with H. pylori on PDE-5i remained at an increased risk of gastric cancer (OR 2.33, 1.11-4.89, p= 0.0218).
Discussion: Our study utilized a comprehensive health network (TriNetX) that evaluated patients on PDE-5 inhibitors (Sildenafil, Tadalafil, and Vardenafil) and found that the rates of developing gastric cancer and colon cancer were lower than those not on these therapies. Additionally, our study highlighted those patients with a prior or current H. pylori infection, despite being on PDE5i, still had an increased risk of gastric cancer. Future prospective studies are needed to further explore the anti-tumor effects of PDE-5 inhibitors on gastric cancer.

Figure: Table 1. Baseline characteristics of patients before and after propensity score matching, including p-values.

Figure: Tables 2 and 3. Outcomes with adjusted OR
Disclosures:
Chisom Nwaneki indicated no relevant financial relationships.
Maurice Facey indicated no relevant financial relationships.
Jaime Perez indicated no relevant financial relationships.
Juanita Merchant indicated no relevant financial relationships.
Fabio Cominelli indicated no relevant financial relationships.
Bianca Islam indicated no relevant financial relationships.
Chisom Nwaneki, MD, MPH1, Maurice Facey, MD2, Jaime A. Perez, PhD3, Juanita L.. Merchant, MD, PhD4, Fabio Cominelli, MD, PhD3, Bianca Islam, MD, PhD3. P4189 - Beyond Erectile Dysfunction: Investigating the Protective Effect of PDE-5 Inhibitors Against Gastric Cancer, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

