Monday Poster Session
Category: Small Intestine
P4125 - Persistent Diarrhea Following CAR-T Therapy for Multiple Myeloma: A Case of Immune Effector Cell-Associated Enterocolitis
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- SR
Stacey Rolak, MD, MPH
Mayo Clinic Arizona
Phoenix, AZ
Presenting Author(s)
Stacey Rolak, MD, MPH1, Chirag Patel, MD1, Jin Cheng, MD, PhD1, Vanessa Eller, MD2, Sarah Umar, MD1
1Mayo Clinic Arizona, Phoenix, AZ; 2University of Arizona, Phoenix, AZ
Introduction: Given the increasing number of patients receiving chimeric antigen receptor T-cell (CAR-T) therapy, it is important to be aware of potential gastrointestinal side effects of this treatment. We highlight a case of immune effector cell (IEC) enterocolitis following CAR-T therapy.
Case Description/
Methods: A 73-year-old female with a history of IgG kappa multiple myeloma status post CAR-T with ciltacabtagene autoleucel was hospitalized 170 days after treatment for acute onset non-bloody diarrhea with >4 L stool per day. She was afebrile and hemodynamically stable. Labs were notable for hypokalemia, hypernatremia, acute kidney injury with creatinine 2.83 mg/dL (from baseline 1.19 mg/dL), albumin 3.4 g/dL. GI pathogen panel and C-diff were negative. Thyroid stimulating hormone, fecal calprotectin, fecal alpha-1 antitrypsin, celiac serologies, and 7AC4 were normal. Colonoscopy was grossly normal with biopsies negative for microscopic colitis or cytomegalovirus. Push enteroscopy demonstrated nodularity in the duodenum and jejunum (Image 1). Small bowel aspirates were negative. Jejunal biopsies demonstrated villous blunting, crypt apoptosis, and patchy areas of increased intraepithelial lymphocytes with a predominance of CD4+ T-cells (Image 2). Genetic analysis demonstrated clonal T-cell receptor gene arrangements. There was no evidence of mass or significant cytological atypia, making findings less consistent with a developing T-cell lymphoma. Her clinical presentation was most consistent with immune effector cell enterocolitis. She received IV methylprednisolone without significant improvement in her symptoms after 5 days, one dose of cyclophosphamide 1.5mg/m2, and given ongoing diarrhea, received two doses of vedolizumab 300mg IV, one week apart. Her diarrhea decreased and she discharged home. She was doing well at follow-up with 2-3 bowel movements per day.
Discussion: IEC enterocolitis is a rare complication of CAR-T therapy, often presenting 1-3 months after infusion. Our patient had no evidence of colonic involvement, and the diagnosis was made based on small intestinal biopsies, highlighting that upper endoscopy with biopsies should be obtained when this condition is suspected. Biopsies demonstrate villous blunting and increased intraepithelial lymphocytes. This condition is treated with systemic corticosteroids, infliximab, or vedolizumab. Developing lymphoproliferative process should remain on the differential diagnosis, especially in patients not responding to treatment.
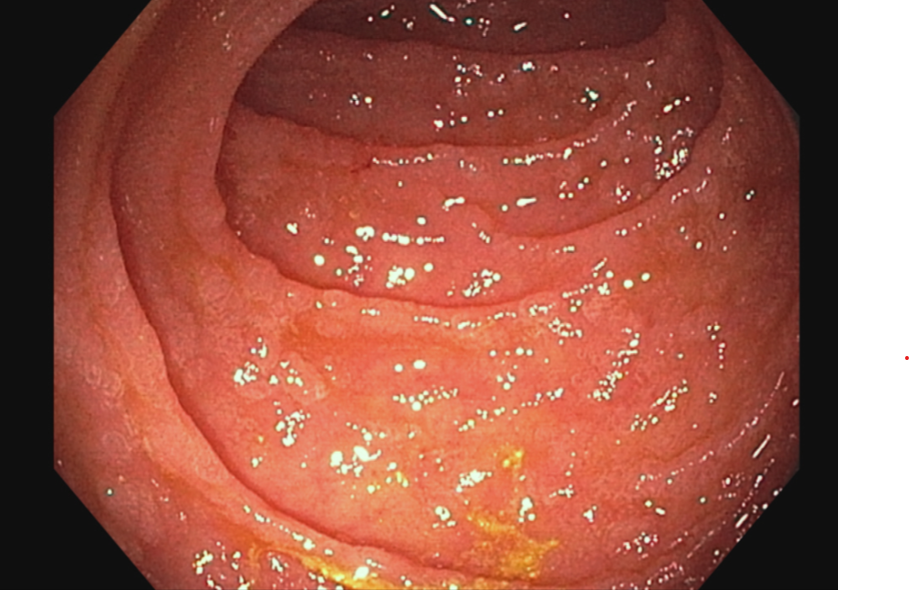
Figure: Upper endoscopy with push enteroscopy demonstrating patchy granularity and nodularity in the duodenum.
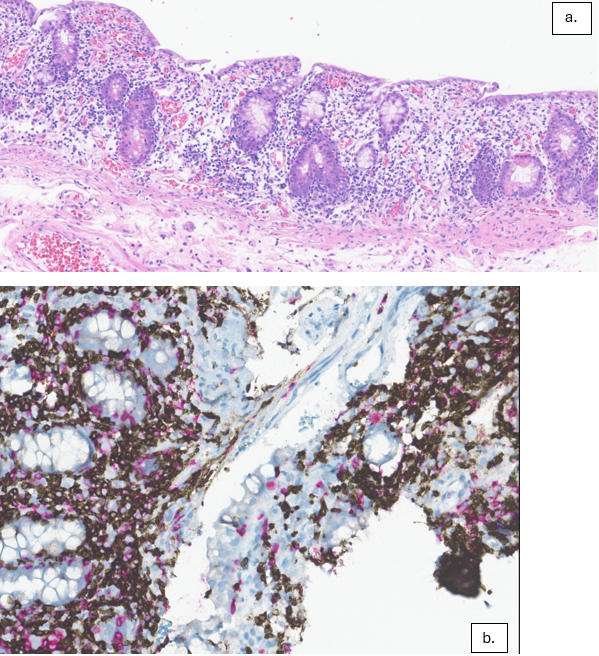
Figure: a. 25x pathology slide of duodenal biopsy demonstrating diffuse villous blunting and paucity of plasma cells within the lamina propria. The epithelium shows an increase in crypt epithelial apoptosis with increased intraepithelial lymphocytosis.
b. 25x pathology slide demonstrating increased intraepithelial lymphocytosis with most T-cells being CD4 positive with T-cell receptor (TCR) β-chain constant region 1 restriction. Polymerase chain reaction study for clonal T-cell gene rearrangement was positive.
Disclosures:
Stacey Rolak indicated no relevant financial relationships.
Chirag Patel: Roche – Advisory Committee/Board Member.
Jin Cheng indicated no relevant financial relationships.
Vanessa Eller indicated no relevant financial relationships.
Sarah Umar indicated no relevant financial relationships.
Stacey Rolak, MD, MPH1, Chirag Patel, MD1, Jin Cheng, MD, PhD1, Vanessa Eller, MD2, Sarah Umar, MD1. P4125 - Persistent Diarrhea Following CAR-T Therapy for Multiple Myeloma: A Case of Immune Effector Cell-Associated Enterocolitis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Mayo Clinic Arizona, Phoenix, AZ; 2University of Arizona, Phoenix, AZ
Introduction: Given the increasing number of patients receiving chimeric antigen receptor T-cell (CAR-T) therapy, it is important to be aware of potential gastrointestinal side effects of this treatment. We highlight a case of immune effector cell (IEC) enterocolitis following CAR-T therapy.
Case Description/
Methods: A 73-year-old female with a history of IgG kappa multiple myeloma status post CAR-T with ciltacabtagene autoleucel was hospitalized 170 days after treatment for acute onset non-bloody diarrhea with >4 L stool per day. She was afebrile and hemodynamically stable. Labs were notable for hypokalemia, hypernatremia, acute kidney injury with creatinine 2.83 mg/dL (from baseline 1.19 mg/dL), albumin 3.4 g/dL. GI pathogen panel and C-diff were negative. Thyroid stimulating hormone, fecal calprotectin, fecal alpha-1 antitrypsin, celiac serologies, and 7AC4 were normal. Colonoscopy was grossly normal with biopsies negative for microscopic colitis or cytomegalovirus. Push enteroscopy demonstrated nodularity in the duodenum and jejunum (Image 1). Small bowel aspirates were negative. Jejunal biopsies demonstrated villous blunting, crypt apoptosis, and patchy areas of increased intraepithelial lymphocytes with a predominance of CD4+ T-cells (Image 2). Genetic analysis demonstrated clonal T-cell receptor gene arrangements. There was no evidence of mass or significant cytological atypia, making findings less consistent with a developing T-cell lymphoma. Her clinical presentation was most consistent with immune effector cell enterocolitis. She received IV methylprednisolone without significant improvement in her symptoms after 5 days, one dose of cyclophosphamide 1.5mg/m2, and given ongoing diarrhea, received two doses of vedolizumab 300mg IV, one week apart. Her diarrhea decreased and she discharged home. She was doing well at follow-up with 2-3 bowel movements per day.
Discussion: IEC enterocolitis is a rare complication of CAR-T therapy, often presenting 1-3 months after infusion. Our patient had no evidence of colonic involvement, and the diagnosis was made based on small intestinal biopsies, highlighting that upper endoscopy with biopsies should be obtained when this condition is suspected. Biopsies demonstrate villous blunting and increased intraepithelial lymphocytes. This condition is treated with systemic corticosteroids, infliximab, or vedolizumab. Developing lymphoproliferative process should remain on the differential diagnosis, especially in patients not responding to treatment.

Figure: Upper endoscopy with push enteroscopy demonstrating patchy granularity and nodularity in the duodenum.

Figure: a. 25x pathology slide of duodenal biopsy demonstrating diffuse villous blunting and paucity of plasma cells within the lamina propria. The epithelium shows an increase in crypt epithelial apoptosis with increased intraepithelial lymphocytosis.
b. 25x pathology slide demonstrating increased intraepithelial lymphocytosis with most T-cells being CD4 positive with T-cell receptor (TCR) β-chain constant region 1 restriction. Polymerase chain reaction study for clonal T-cell gene rearrangement was positive.
Disclosures:
Stacey Rolak indicated no relevant financial relationships.
Chirag Patel: Roche – Advisory Committee/Board Member.
Jin Cheng indicated no relevant financial relationships.
Vanessa Eller indicated no relevant financial relationships.
Sarah Umar indicated no relevant financial relationships.
Stacey Rolak, MD, MPH1, Chirag Patel, MD1, Jin Cheng, MD, PhD1, Vanessa Eller, MD2, Sarah Umar, MD1. P4125 - Persistent Diarrhea Following CAR-T Therapy for Multiple Myeloma: A Case of Immune Effector Cell-Associated Enterocolitis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
