Monday Poster Session
Category: Small Intestine
P4110 - Small Bowel Obstruction Associated With Semaglutide Use
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

James E. McSweeney, III, MD
South Georgia Medical Center
Valdosta, GA
Presenting Author(s)
James E. McSweeney, MD1, Amrendra Mandal, MD1, Katherine Waters, BS2, Nathaniel A. Kirchoff, BS3
1South Georgia Medical Center, Valdosta, GA; 2South Georgia Medical Center, Macon, GA; 3South Georgia Medical Center, Savannah, GA
Introduction: Glucagon-like peptide-1 receptor agonists are FDA-approved medications initially developed for type 2 diabetes but have recently gained popularity due to their weight loss benefits. While beneficial for weight management delayed gastric emptying can lead to gastrointestinal adverse effects including nausea, vomiting, constipation, gastroparesis and rarely small bowel obstruction1,2. Given the increasing popularity of GLP-1 agonists; how can clinicians better identify patients at increased risk for severe gastrointestinal complications such as small bowel obstruction?
Case Description/
Methods: A 66-year-old female with a medical history significant for obesity presented to the ER with a two-day history of abdominal pain and absence of flatus or bowel movements. Two months ago she was initiated on treatment with semaglutide for weight loss. Her surgical history includes a hysterectomy. Despite NG tube decompression she continued to have a surgical abdomen. Exploratory laparotomy revealed congested bowel with early ischemic changes necessitating a 10 cm small bowel resection. Approximately one year later while still using semaglutide she presented again with abdominal pain. Exploratory laparotomy was performed revealing extensive adhesions requiring lysis and additional small bowel resection with anastomosis. Following the second surgical intervention discontinuation of semaglutide was recommended by her general surgeon as a likely etiology behind her recurrent small bowel obstruction.
Discussion: GLP-1 receptor agonists have been associated with bowel obstruction at rates approximately 4.5 times higher than other antidiabetic medications1,2. This case underscores the importance of recognizing bowel obstruction as a potentially significant adverse effect of GLP-1 receptor agonists. Clinicians should maintain vigilance when initiating and continuing GLP medications especially in patients with prior abdominal surgeries.
References:
1. Faillie, J.-L., Yin, H., Yu, O. H. Y., Herrero, A., Altwegg, R., Renoux, C., & Azoulay, L. (2022). Incretin-Based Drugs and Risk of Intestinal Obstruction Among Patients With Type 2 Diabetes. Clinical Pharmacology & Therapeutics, 111(1), 272–282.
2. Gudin, B., Ladhari, C., Robin, P., Laroche, M.-L., Babai, S., Hillaire-Buys, D., & Faillie, J.-L. (2020). Incretin-based drugs and intestinal obstruction: A pharmacovigilance study. Therapies, 75(6), 641–647.
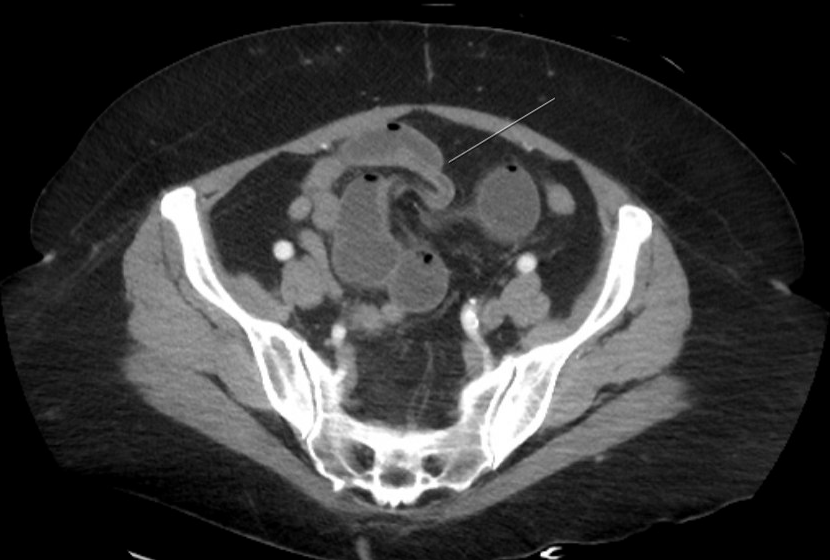
Figure: Fig.1 Coronal section of CT abdomen/pelvis. There is trace ascites in the pelvis. There is no evidence of free air. Dilated bowel extends into the pelvis.
Disclosures:
James McSweeney indicated no relevant financial relationships.
Amrendra Mandal indicated no relevant financial relationships.
Katherine Waters indicated no relevant financial relationships.
Nathaniel Kirchoff indicated no relevant financial relationships.
James E. McSweeney, MD1, Amrendra Mandal, MD1, Katherine Waters, BS2, Nathaniel A. Kirchoff, BS3. P4110 - Small Bowel Obstruction Associated With Semaglutide Use, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1South Georgia Medical Center, Valdosta, GA; 2South Georgia Medical Center, Macon, GA; 3South Georgia Medical Center, Savannah, GA
Introduction: Glucagon-like peptide-1 receptor agonists are FDA-approved medications initially developed for type 2 diabetes but have recently gained popularity due to their weight loss benefits. While beneficial for weight management delayed gastric emptying can lead to gastrointestinal adverse effects including nausea, vomiting, constipation, gastroparesis and rarely small bowel obstruction1,2. Given the increasing popularity of GLP-1 agonists; how can clinicians better identify patients at increased risk for severe gastrointestinal complications such as small bowel obstruction?
Case Description/
Methods: A 66-year-old female with a medical history significant for obesity presented to the ER with a two-day history of abdominal pain and absence of flatus or bowel movements. Two months ago she was initiated on treatment with semaglutide for weight loss. Her surgical history includes a hysterectomy. Despite NG tube decompression she continued to have a surgical abdomen. Exploratory laparotomy revealed congested bowel with early ischemic changes necessitating a 10 cm small bowel resection. Approximately one year later while still using semaglutide she presented again with abdominal pain. Exploratory laparotomy was performed revealing extensive adhesions requiring lysis and additional small bowel resection with anastomosis. Following the second surgical intervention discontinuation of semaglutide was recommended by her general surgeon as a likely etiology behind her recurrent small bowel obstruction.
Discussion: GLP-1 receptor agonists have been associated with bowel obstruction at rates approximately 4.5 times higher than other antidiabetic medications1,2. This case underscores the importance of recognizing bowel obstruction as a potentially significant adverse effect of GLP-1 receptor agonists. Clinicians should maintain vigilance when initiating and continuing GLP medications especially in patients with prior abdominal surgeries.
References:
1. Faillie, J.-L., Yin, H., Yu, O. H. Y., Herrero, A., Altwegg, R., Renoux, C., & Azoulay, L. (2022). Incretin-Based Drugs and Risk of Intestinal Obstruction Among Patients With Type 2 Diabetes. Clinical Pharmacology & Therapeutics, 111(1), 272–282.
2. Gudin, B., Ladhari, C., Robin, P., Laroche, M.-L., Babai, S., Hillaire-Buys, D., & Faillie, J.-L. (2020). Incretin-based drugs and intestinal obstruction: A pharmacovigilance study. Therapies, 75(6), 641–647.

Figure: Fig.1 Coronal section of CT abdomen/pelvis. There is trace ascites in the pelvis. There is no evidence of free air. Dilated bowel extends into the pelvis.
Disclosures:
James McSweeney indicated no relevant financial relationships.
Amrendra Mandal indicated no relevant financial relationships.
Katherine Waters indicated no relevant financial relationships.
Nathaniel Kirchoff indicated no relevant financial relationships.
James E. McSweeney, MD1, Amrendra Mandal, MD1, Katherine Waters, BS2, Nathaniel A. Kirchoff, BS3. P4110 - Small Bowel Obstruction Associated With Semaglutide Use, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
