Monday Poster Session
Category: Small Intestine
P4077 - Evaluating the Use of Latiglutenase as Gluten-Targeting Protease in Patients With Celiac Disease: A Meta-Analysis of Randomized Controlled Trials
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- MA
Mageda Al Areqi, MD
Raritan Bay Medical Center
Perth Amboy, NJ
Presenting Author(s)
Rana H. Shembesh, MBBCh1, Abdallah Khashan, MD2, Mohammed S. Beshr, MBBS3, Mageda Al Areqi, MD4, Bisher Sawaf, MD5, Muhammed Elhadi, MD6
1Libyan International Medical University, Faculty of Medicine, Benghazi, Benghazi, Libya; 2Capital Health Regional Medical Center, Trenton, NJ; 3Sana’a University, Faculty of Medicine and Health Sciences, Sana'a, Hadramawt, Yemen; 4Raritan Bay Medical Center, Perth Amboy, NJ; 5University of Toledo Medical Center, Toledo, OH; 6College of Medicine, Korea University, Seongbuk, Seoul-t'ukpyolsi, Republic of Korea
Introduction: A gluten-free diet remains the treatment of choice for celiac disease, but due to cross-contamination and the difficulty in adherence, celiac symptoms do emerge. New pharmacological options are under investigation, and Latiglutenase, a new investigational drug, is a recombinant protein with gluten-targeting protease that degrades gluten molecules.
Methods: On November 17, 2024, related articles were searched in the following databases: PubMed, Scopus, and Cochrane. Our inclusion criteria were only randomized controlled clinical trials that evaluated the use of Latiglutenase in celiac patients. Our outcomes were the mean change in villus height to crypt depth ratio to assess histologic impact of the treatment in villous atrophy, the mean change in the density of intraepithelial lymphocytes to assess inflammatory changes with the treatment, and the difference in the symptoms related to celiac disease. In this meta-analysis, we used the mean difference and the odds ratio (OR) with the 95% confidence interval to estimate the effect size. A random-effects model was applied.
Results: Out of 143 articles screened, only 3 were eligible for inclusion, with a total of 253 patients. All included patients were on a strict gluten-free diet for at least one year prior to the trials. Two studies (Murray 2022 and Lahdeaho) administered a daily gluten challenge of approximately 2 grams for 6 weeks, along a gluten-free diet. In our analysis of the mean change in the villus height to crypt depth ratio, there was no statistical difference between the groups, with a mean difference of 0.05 (CI: -0.41 to 0.51, p = 0.82). The mean change in the density of intraepithelial lymphocytes also failed to show any statistical difference, with a mean difference of -11.82 (CI: -28.41 to 4.78, p = 0.16). Symptoms such as nausea, diarrhea, and abdominal distention showed no significant difference between the treatment groups, as all shared a p-value above 0.05. Finally, there were no statistical differences in the rates of any adverse events between the two groups with an odds ratio of 0.79 (CI: 0.45 to 1.39, p = 0.42).
Discussion: Latiglutenase has failed to show a meaningful difference in the histologic markers when compared to a placebo, indicating no impact on villous atrophy. Similar results were observed for symptoms related to celiac disease. There is a need for non-dietary pharmacological options for celiac disease.
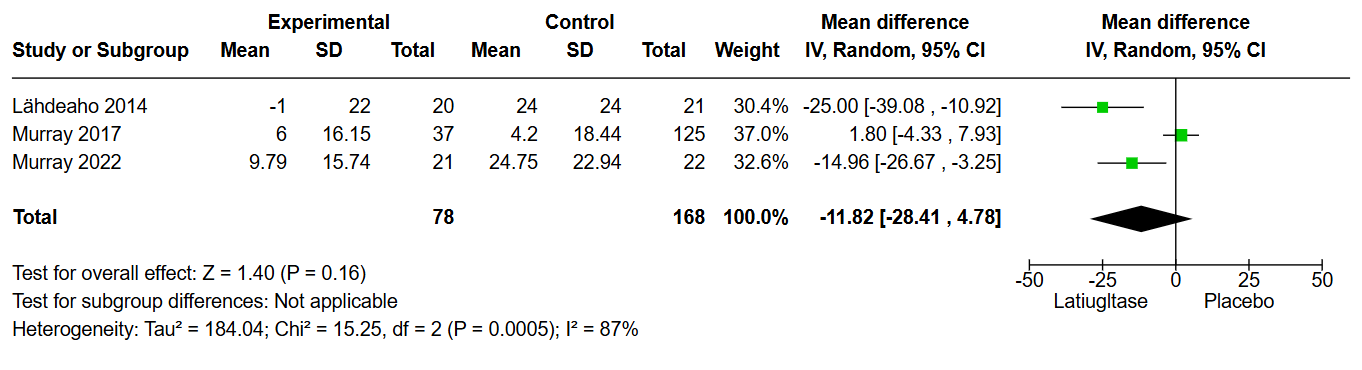
Figure: Figure 1: Change in the inter-epithelial Lymphocytes

Figure: Figure 2: GI Symptoms
Disclosures:
Rana Shembesh indicated no relevant financial relationships.
Abdallah Khashan indicated no relevant financial relationships.
Mohammed Beshr indicated no relevant financial relationships.
Mageda Al Areqi indicated no relevant financial relationships.
Bisher Sawaf indicated no relevant financial relationships.
Muhammed Elhadi indicated no relevant financial relationships.
Rana H. Shembesh, MBBCh1, Abdallah Khashan, MD2, Mohammed S. Beshr, MBBS3, Mageda Al Areqi, MD4, Bisher Sawaf, MD5, Muhammed Elhadi, MD6. P4077 - Evaluating the Use of Latiglutenase as Gluten-Targeting Protease in Patients With Celiac Disease: A Meta-Analysis of Randomized Controlled Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Libyan International Medical University, Faculty of Medicine, Benghazi, Benghazi, Libya; 2Capital Health Regional Medical Center, Trenton, NJ; 3Sana’a University, Faculty of Medicine and Health Sciences, Sana'a, Hadramawt, Yemen; 4Raritan Bay Medical Center, Perth Amboy, NJ; 5University of Toledo Medical Center, Toledo, OH; 6College of Medicine, Korea University, Seongbuk, Seoul-t'ukpyolsi, Republic of Korea
Introduction: A gluten-free diet remains the treatment of choice for celiac disease, but due to cross-contamination and the difficulty in adherence, celiac symptoms do emerge. New pharmacological options are under investigation, and Latiglutenase, a new investigational drug, is a recombinant protein with gluten-targeting protease that degrades gluten molecules.
Methods: On November 17, 2024, related articles were searched in the following databases: PubMed, Scopus, and Cochrane. Our inclusion criteria were only randomized controlled clinical trials that evaluated the use of Latiglutenase in celiac patients. Our outcomes were the mean change in villus height to crypt depth ratio to assess histologic impact of the treatment in villous atrophy, the mean change in the density of intraepithelial lymphocytes to assess inflammatory changes with the treatment, and the difference in the symptoms related to celiac disease. In this meta-analysis, we used the mean difference and the odds ratio (OR) with the 95% confidence interval to estimate the effect size. A random-effects model was applied.
Results: Out of 143 articles screened, only 3 were eligible for inclusion, with a total of 253 patients. All included patients were on a strict gluten-free diet for at least one year prior to the trials. Two studies (Murray 2022 and Lahdeaho) administered a daily gluten challenge of approximately 2 grams for 6 weeks, along a gluten-free diet. In our analysis of the mean change in the villus height to crypt depth ratio, there was no statistical difference between the groups, with a mean difference of 0.05 (CI: -0.41 to 0.51, p = 0.82). The mean change in the density of intraepithelial lymphocytes also failed to show any statistical difference, with a mean difference of -11.82 (CI: -28.41 to 4.78, p = 0.16). Symptoms such as nausea, diarrhea, and abdominal distention showed no significant difference between the treatment groups, as all shared a p-value above 0.05. Finally, there were no statistical differences in the rates of any adverse events between the two groups with an odds ratio of 0.79 (CI: 0.45 to 1.39, p = 0.42).
Discussion: Latiglutenase has failed to show a meaningful difference in the histologic markers when compared to a placebo, indicating no impact on villous atrophy. Similar results were observed for symptoms related to celiac disease. There is a need for non-dietary pharmacological options for celiac disease.

Figure: Figure 1: Change in the inter-epithelial Lymphocytes

Figure: Figure 2: GI Symptoms
Disclosures:
Rana Shembesh indicated no relevant financial relationships.
Abdallah Khashan indicated no relevant financial relationships.
Mohammed Beshr indicated no relevant financial relationships.
Mageda Al Areqi indicated no relevant financial relationships.
Bisher Sawaf indicated no relevant financial relationships.
Muhammed Elhadi indicated no relevant financial relationships.
Rana H. Shembesh, MBBCh1, Abdallah Khashan, MD2, Mohammed S. Beshr, MBBS3, Mageda Al Areqi, MD4, Bisher Sawaf, MD5, Muhammed Elhadi, MD6. P4077 - Evaluating the Use of Latiglutenase as Gluten-Targeting Protease in Patients With Celiac Disease: A Meta-Analysis of Randomized Controlled Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

