Monday Poster Session
Category: Small Intestine
P4070 - Immune Checkpoint Inhibitors and the Risk of Celiac Disease: A Propensity Score-Matched Analysis
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- NW
Niven Wang, DO
University of New Mexico
Albuquerque, NM
Presenting Author(s)
Niven Wang, DO1, Abdelrahman Yousef, MD2, Daniel Paverini, MD1, Christopher Chang, MD1
1University of New Mexico, Albuquerque, NM; 2University of New Mexico Hospital, Albuquerque, NM
Introduction: Immune checkpoint inhibitors (ICIs) are increasingly used to treat advanced malignancies but are associated with immune-related adverse events (irAEs), particularly gastrointestinal complications. While ICI-related colitis is well documented, the risk of developing celiac disease (CeD), an immune-mediated enteropathy, remains unclear.
Methods: We conducted a retrospective cohort study using a large, de-identified electronic health record database (TriNetX). Adult patients with malignant neoplasms (ICD-10: C00–C96) were included. The ICI group received atezolizumab, nivolumab, durvalumab, ipilimumab, or pembrolizumab; the non-ICI group had no exposure to these agents. Patients with pre-existing CeD were excluded. Propensity score matching (1:1) was performed based on age, sex, and cancer type, yielding two matched cohorts (n=124,205 each). The primary outcome was new-onset CeD (ICD-10: K90.0) within 2 years of cancer diagnosis and ICI initiation.
Results: In the matched analysis, CeD developed in 125 patients treated with ICIs (0.10%) versus 69 patients in the non-ICI group (0.06%). This corresponds to a risk ratio of 1.81 (95% CI: 1.35–2.43, p < 0.001). While the average number of CeD-related encounters was higher in the ICI group (mean 4.1 vs. 3.1), this difference did not reach statistical significance (p = 0.285). The findings suggest that ICIs are associated with nearly double the risk of developing CeD compared to patients not exposed to these agents.
Discussion: Our results highlight a potential new immune-related adverse event associated with ICIs—celiac disease. Although rare, CeD may be under-recognized in this setting. Oncologists and gastroenterologists should consider CeD in patients presenting with persistent mild diarrhea, weight loss, or nutritional deficiencies while on ICIs. Further prospective studies are needed to understand the mechanisms and develop appropriate screening strategies.
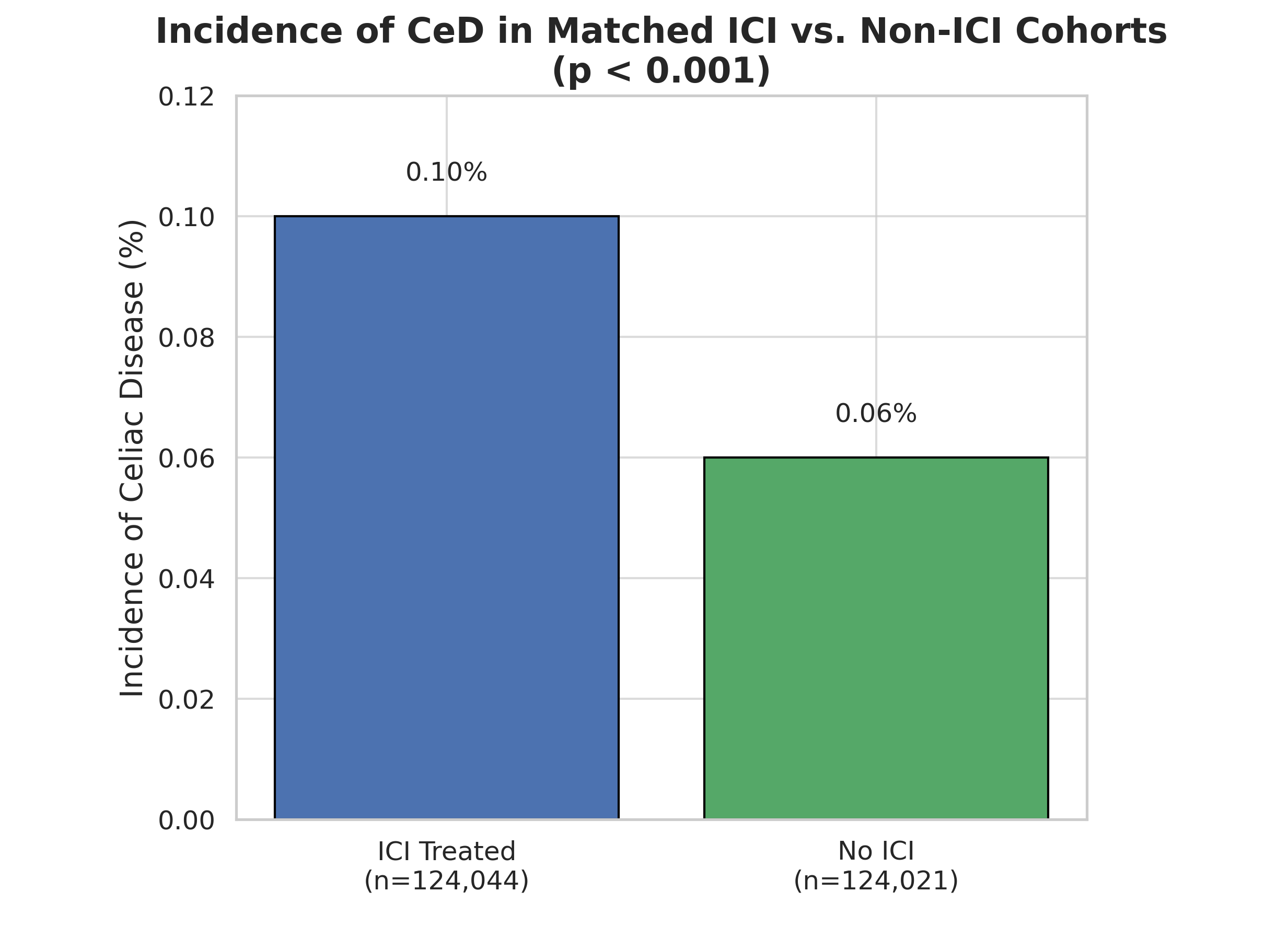
Figure: Incidence of Celiac Disease in patients treated with ICIs compared to cohort not exposed to ICI
Disclosures:
Niven Wang indicated no relevant financial relationships.
Abdelrahman Yousef indicated no relevant financial relationships.
Daniel Paverini indicated no relevant financial relationships.
Christopher Chang indicated no relevant financial relationships.
Niven Wang, DO1, Abdelrahman Yousef, MD2, Daniel Paverini, MD1, Christopher Chang, MD1. P4070 - Immune Checkpoint Inhibitors and the Risk of Celiac Disease: A Propensity Score-Matched Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of New Mexico, Albuquerque, NM; 2University of New Mexico Hospital, Albuquerque, NM
Introduction: Immune checkpoint inhibitors (ICIs) are increasingly used to treat advanced malignancies but are associated with immune-related adverse events (irAEs), particularly gastrointestinal complications. While ICI-related colitis is well documented, the risk of developing celiac disease (CeD), an immune-mediated enteropathy, remains unclear.
Methods: We conducted a retrospective cohort study using a large, de-identified electronic health record database (TriNetX). Adult patients with malignant neoplasms (ICD-10: C00–C96) were included. The ICI group received atezolizumab, nivolumab, durvalumab, ipilimumab, or pembrolizumab; the non-ICI group had no exposure to these agents. Patients with pre-existing CeD were excluded. Propensity score matching (1:1) was performed based on age, sex, and cancer type, yielding two matched cohorts (n=124,205 each). The primary outcome was new-onset CeD (ICD-10: K90.0) within 2 years of cancer diagnosis and ICI initiation.
Results: In the matched analysis, CeD developed in 125 patients treated with ICIs (0.10%) versus 69 patients in the non-ICI group (0.06%). This corresponds to a risk ratio of 1.81 (95% CI: 1.35–2.43, p < 0.001). While the average number of CeD-related encounters was higher in the ICI group (mean 4.1 vs. 3.1), this difference did not reach statistical significance (p = 0.285). The findings suggest that ICIs are associated with nearly double the risk of developing CeD compared to patients not exposed to these agents.
Discussion: Our results highlight a potential new immune-related adverse event associated with ICIs—celiac disease. Although rare, CeD may be under-recognized in this setting. Oncologists and gastroenterologists should consider CeD in patients presenting with persistent mild diarrhea, weight loss, or nutritional deficiencies while on ICIs. Further prospective studies are needed to understand the mechanisms and develop appropriate screening strategies.

Figure: Incidence of Celiac Disease in patients treated with ICIs compared to cohort not exposed to ICI
Disclosures:
Niven Wang indicated no relevant financial relationships.
Abdelrahman Yousef indicated no relevant financial relationships.
Daniel Paverini indicated no relevant financial relationships.
Christopher Chang indicated no relevant financial relationships.
Niven Wang, DO1, Abdelrahman Yousef, MD2, Daniel Paverini, MD1, Christopher Chang, MD1. P4070 - Immune Checkpoint Inhibitors and the Risk of Celiac Disease: A Propensity Score-Matched Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
