Monday Poster Session
Category: Small Intestine
P4066 - Treatment Strategies for Refractory Celiac Disease: A Systematic Review and Meta-Analysis
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- OA
Omar Al Ta'ani, MD
Department of Internal Medicine, Allegheny Health Network, Pittsburgh, Pennsylvania, USA
Pittsburgh, PA
Presenting Author(s)
Omar Al Ta’ani, MD1, Yahya Alhalalmeh, MD2, Angela Hardi, 3, Osama Altayar, MD3
1Department of Internal Medicine, Allegheny Health Network, Pittsburgh, Pennsylvania, USA, Pittsburgh, PA; 2New York Medical College - Saint Michael's Medical Center, Newark, NJ; 3Washington University School of Medicine in St. Louis, St. Louis, MO
Introduction: Refractory celiac disease (RCD) is a severe complication of celiac disease defined by persistent symptoms and intestinal damage despite strict adherence to a gluten-free diet. With no FDA-approved therapies and limited evidence, treatment remains challenging. This systematic review and meta-analysis evaluated current treatment strategies for RCD to guide clinical care.
Methods: We systematically searched MEDLINE, Embase, Scopus, the Cochrane Library, and ClinicalTrials.gov through November 2024 for studies evaluating therapeutic interventions in RCD, including clinical trials, cohort studies, and case series with ≥5 patients. The main outcomes were clinical response, defined as improvement in symptoms, and clinical remission, defined as sustained complete resolution of symptoms. Pooled proportions were estimated using a fixed-effects model.
Results: 32 studies (1,038 patients) were included, with 5 focusing solely on type I RCD, 11 on type II RCD, and 16 reporting on both subtypes. In patients with RCD I (Figure 1), budesonide led to clinical response in 88% (95% CI, 82–94%; N/n = 6/144; I² = 61.8%) and remission in 65% (55–73%; N/n = 6/119; I² = 82.8%) of patients. Open-capsule budesonide led to clinical response in 87% (80–93%; N/n = 3/110; I² = 71.6%) and remission in 60% (51–69%; N/n = 3/110; I² = 74.4%). A clinical response was achieved in 97% (77–100%; N/n = 3/16; I² = 0.0%) and 100% (91–100%; N/n = 2/11; I² = 0.0%) of patients that received systematic steroids alone and combined with azathioprine, consecutively.
In patients with RCD II (Figure 2), budesonide was associated with a clinical response in 96% (83–100%; N/n = 4/32; I² = 44.7%) and remission in 27% (10–46%; N/n = 3/28; I² = 91.2%). Systemic steroids led to a response in 76% (60–89%; N/n = 2/40; I² = 0.0%) and remission in 6% (0–17%; N/n = 2/40; I² = 0.0%) of patients. Combination therapy resulted in clinical response in 100% (94–100%; N/n = 3/27; I² = 12.3%) and remission in 79% (56–97%; N/n = 3/27; I² = 95.1%). Cladribine demonstrated response in 71% (57–83%) and remission in 45% (27–64%; N/n = 2/34; I² = 81.4%) of patients, while stem cell transplantation led to response in 78% (56–95%; N/n = 3/29; I² = 3.9%) and remission in 67% (33–95%; N/n = 2/16; I² = 0.0%).
Discussion: RCD I often responds well to budesonide and systemic steroids, whereas RCD II poses a therapeutic challenge with limited effective options. Further studies are needed to validate emerging therapies and develop standardized treatment algorithms.
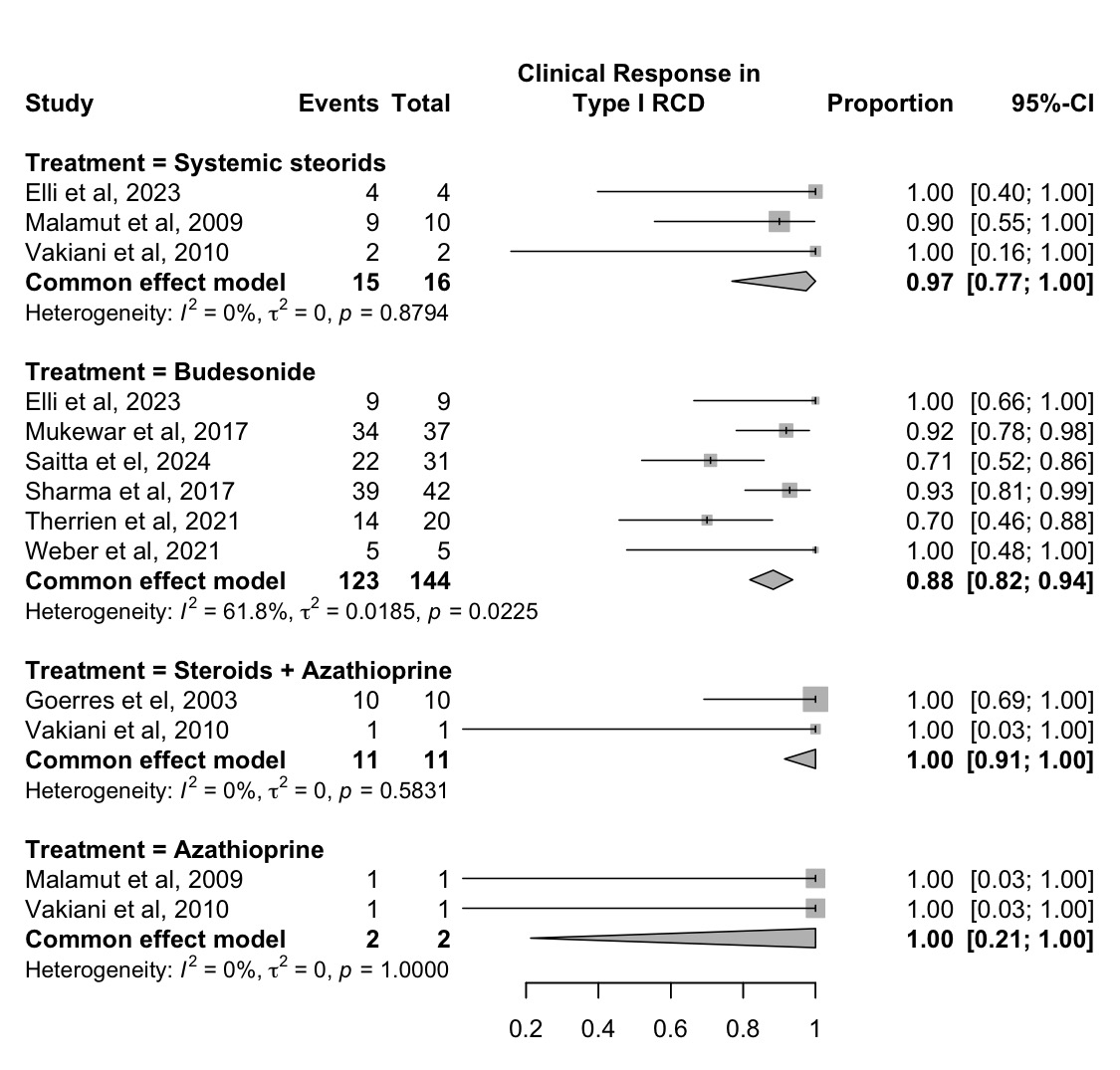
Figure: Figure 1. Proportion of patients with type I refractory celiac disease achieving any clinical response, stratified by treatment modality.
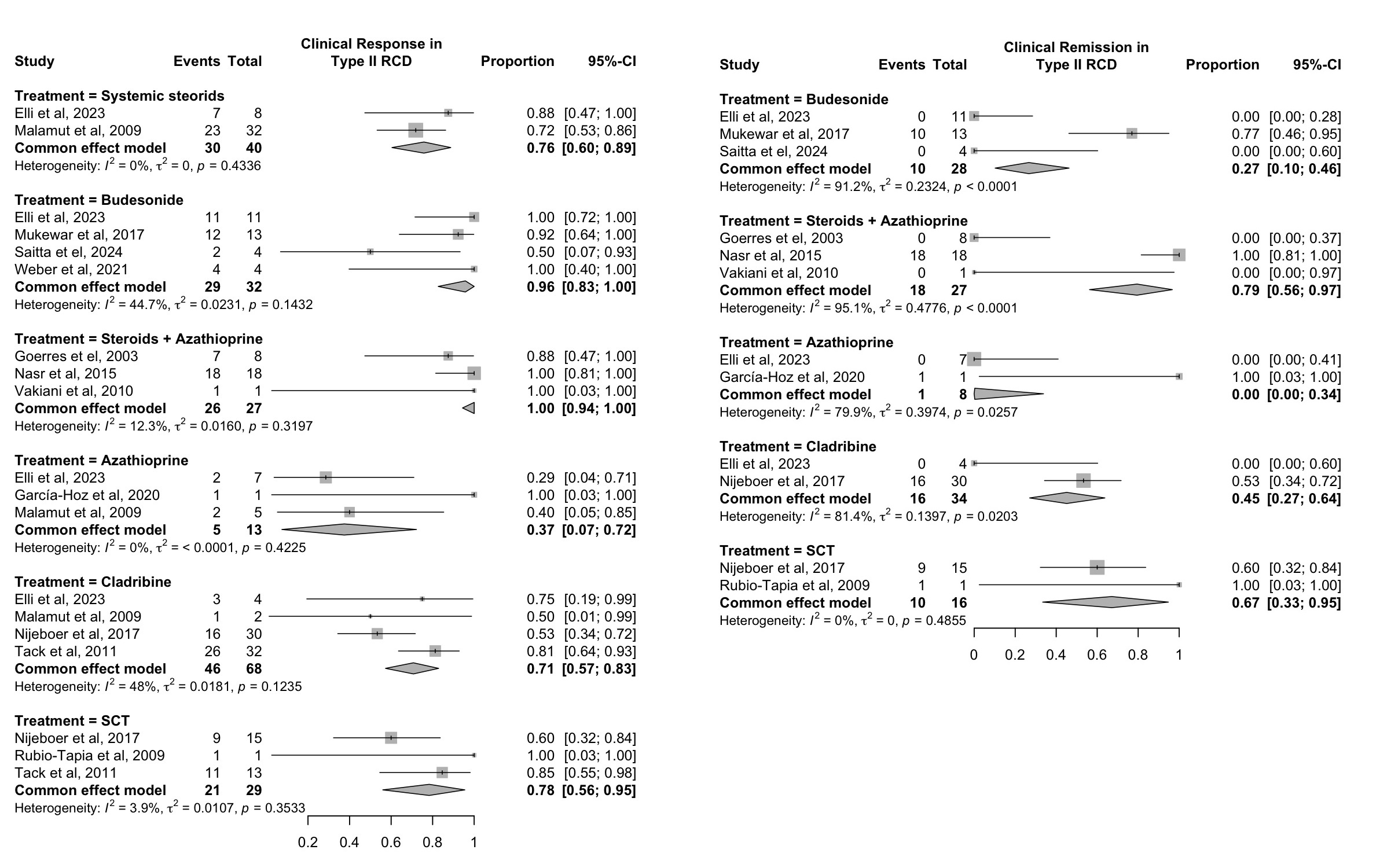
Figure: Figure 2. Proportion of patients with type II refractory celiac disease achieving any clinical response or complete clinical remission, stratified by treatment modality.
Disclosures:
Omar Al Ta’ani indicated no relevant financial relationships.
Yahya Alhalalmeh indicated no relevant financial relationships.
Angela Hardi indicated no relevant financial relationships.
Osama Altayar indicated no relevant financial relationships.
Omar Al Ta’ani, MD1, Yahya Alhalalmeh, MD2, Angela Hardi, 3, Osama Altayar, MD3. P4066 - Treatment Strategies for Refractory Celiac Disease: A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Department of Internal Medicine, Allegheny Health Network, Pittsburgh, Pennsylvania, USA, Pittsburgh, PA; 2New York Medical College - Saint Michael's Medical Center, Newark, NJ; 3Washington University School of Medicine in St. Louis, St. Louis, MO
Introduction: Refractory celiac disease (RCD) is a severe complication of celiac disease defined by persistent symptoms and intestinal damage despite strict adherence to a gluten-free diet. With no FDA-approved therapies and limited evidence, treatment remains challenging. This systematic review and meta-analysis evaluated current treatment strategies for RCD to guide clinical care.
Methods: We systematically searched MEDLINE, Embase, Scopus, the Cochrane Library, and ClinicalTrials.gov through November 2024 for studies evaluating therapeutic interventions in RCD, including clinical trials, cohort studies, and case series with ≥5 patients. The main outcomes were clinical response, defined as improvement in symptoms, and clinical remission, defined as sustained complete resolution of symptoms. Pooled proportions were estimated using a fixed-effects model.
Results: 32 studies (1,038 patients) were included, with 5 focusing solely on type I RCD, 11 on type II RCD, and 16 reporting on both subtypes. In patients with RCD I (Figure 1), budesonide led to clinical response in 88% (95% CI, 82–94%; N/n = 6/144; I² = 61.8%) and remission in 65% (55–73%; N/n = 6/119; I² = 82.8%) of patients. Open-capsule budesonide led to clinical response in 87% (80–93%; N/n = 3/110; I² = 71.6%) and remission in 60% (51–69%; N/n = 3/110; I² = 74.4%). A clinical response was achieved in 97% (77–100%; N/n = 3/16; I² = 0.0%) and 100% (91–100%; N/n = 2/11; I² = 0.0%) of patients that received systematic steroids alone and combined with azathioprine, consecutively.
In patients with RCD II (Figure 2), budesonide was associated with a clinical response in 96% (83–100%; N/n = 4/32; I² = 44.7%) and remission in 27% (10–46%; N/n = 3/28; I² = 91.2%). Systemic steroids led to a response in 76% (60–89%; N/n = 2/40; I² = 0.0%) and remission in 6% (0–17%; N/n = 2/40; I² = 0.0%) of patients. Combination therapy resulted in clinical response in 100% (94–100%; N/n = 3/27; I² = 12.3%) and remission in 79% (56–97%; N/n = 3/27; I² = 95.1%). Cladribine demonstrated response in 71% (57–83%) and remission in 45% (27–64%; N/n = 2/34; I² = 81.4%) of patients, while stem cell transplantation led to response in 78% (56–95%; N/n = 3/29; I² = 3.9%) and remission in 67% (33–95%; N/n = 2/16; I² = 0.0%).
Discussion: RCD I often responds well to budesonide and systemic steroids, whereas RCD II poses a therapeutic challenge with limited effective options. Further studies are needed to validate emerging therapies and develop standardized treatment algorithms.

Figure: Figure 1. Proportion of patients with type I refractory celiac disease achieving any clinical response, stratified by treatment modality.

Figure: Figure 2. Proportion of patients with type II refractory celiac disease achieving any clinical response or complete clinical remission, stratified by treatment modality.
Disclosures:
Omar Al Ta’ani indicated no relevant financial relationships.
Yahya Alhalalmeh indicated no relevant financial relationships.
Angela Hardi indicated no relevant financial relationships.
Osama Altayar indicated no relevant financial relationships.
Omar Al Ta’ani, MD1, Yahya Alhalalmeh, MD2, Angela Hardi, 3, Osama Altayar, MD3. P4066 - Treatment Strategies for Refractory Celiac Disease: A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
