Monday Poster Session
Category: Small Intestine
P4053 - Clinical and Psychological Burden Among Patients with Short Bowel Syndrome Requiring Parenteral Support: Findings From a Multinational Survey
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Roopa Vemulapalli, MD
University of Texas Southwestern Medical Center
Dallas, TX
Presenting Author(s)
Marion F.. Winkler, PhD, RD1, Jenny E.. Harrison, MS2, Roopa Vemulapalli, MD3, Vanessa J.. Kumpf, PharmD4, Gail Mitchell, MBA5, Daniel Wolin, BS6, Laurie Zografos, BS7, Mindy Yang, PharmD8, Laurin Jackson, MA7, Jinyi Wang, MS9, Jeff Henderson, BA10, Mena Boules, MD11, Syed-Mohammed Jafri, MD12
1Rhode Island Hospital, Providence, RI; 2Girls With Guts, Turners Falls, MA; 3University of Texas Southwestern Medical Center, Dallas, TX; 4Vanderbilt University Medical Center, Nashville, TN; 5Ironwood Pharmaceuticals, Walchwil, Zug, Switzerland; 6RTI-Health Solutions, Marshall, MI; 7RTI Health Solutions, Research Triangle Park, NC; 8Ironwood Pharmaceuticals, Mount Pocono, PA; 9RTI-HS, Research Triangle Park, NC; 10Ironwood Pharmaceuticals, Jupiter, FL; 11Ironwood Pharmaceuticals, Inc., Boston, MA; 12Henry Ford Health, Detroit, MI
Introduction: Short bowel syndrome (SBS) is a rare, complex condition characterized by reduced intestinal absorptive capacity. Many patients require long-term parenteral support (PS), including total parenteral nutrition (TPN) and/or intravenous (IV) hydration, to maintain hydration and nutrition. Our study evaluated the clinical and psychological burden associated with SBS and its current management approaches.
Methods: In the context of a noninterventional, cross-sectional, online survey among patients with SBS in the United States (US) and Europe, adults (≥18 years) with SBS dependent on PS provided information on their comorbidities, symptoms or complications of SBS, healthcare resource utilization, and PS use and associated symptoms.
Results: Among 91 patients (50 from the US; 41 from Europe), 69.1% were White, 58.2% were female, and the mean age at SBS diagnosis was 43.5 years. The majority (79.3%-80.6%) reported using TPN and/or IV hydration for over 1 year. Over half (52.7%) reported a stoma/ostomy bag, and 27.8% had a feeding tube. Common comorbidities included Crohn’s disease (25.3%), fistula (13.2%), and ulcerative colitis (11.0%). In the past 6 months, patients frequently reported experiencing fatigue (73.6%), abdominal pain/discomfort (60.4%), anxiety/depression (59.3%), malabsorption/weight loss (52.7%), and dehydration/electrolyte imbalances (51.6%).
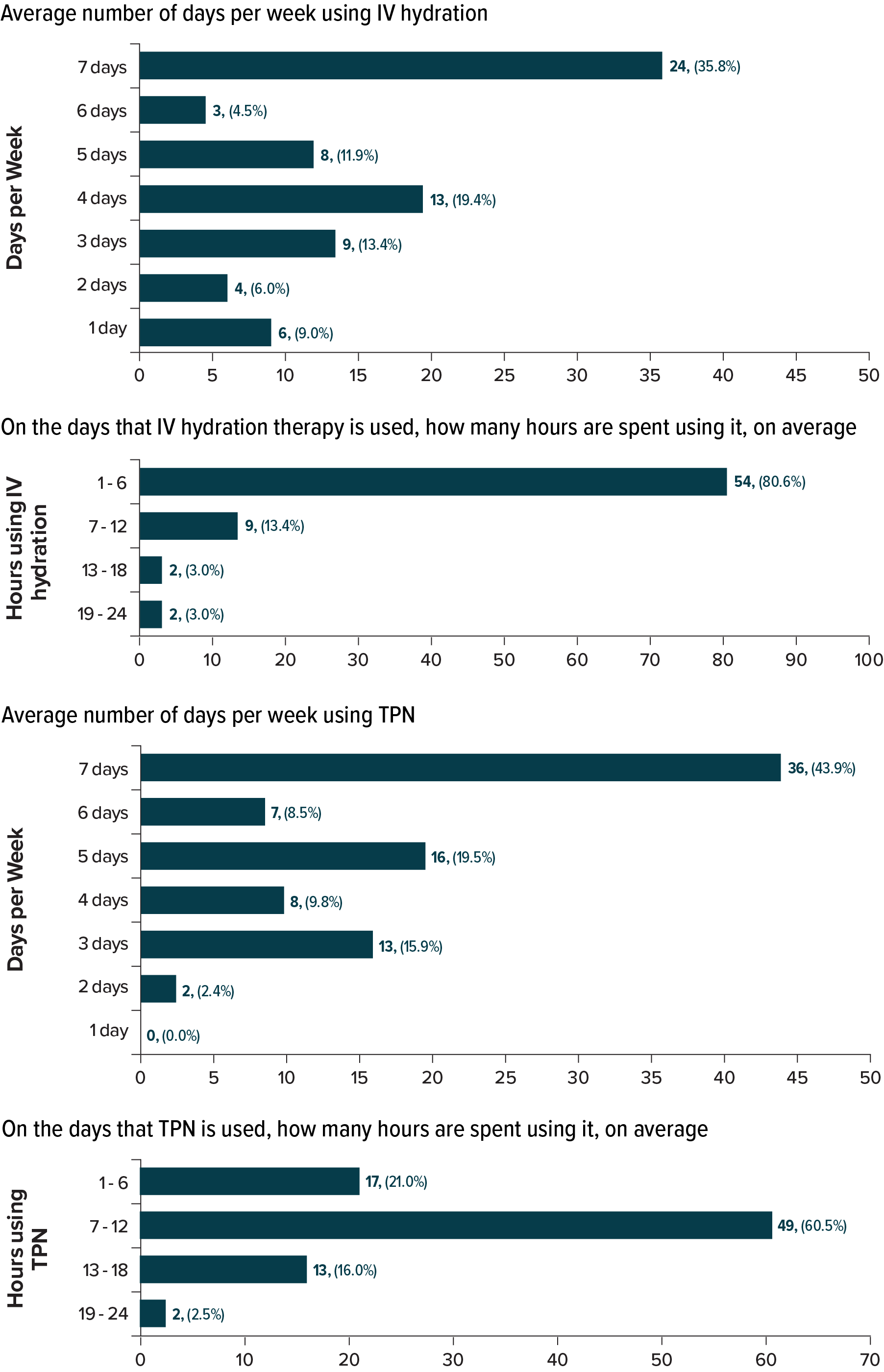
All patients were PS dependent: 26.4% on TPN only, 9.9% on IV hydration only, and 63.7% on both; 24.2% reported current glucagon-like peptide-2 analog use. PS was infused frequently: 72.0% and 52.2% received TPN or IV hydration ≥5 days per week, respectively. Daily treatment duration averaged 10.1 (standard deviation [SD], 4.2) hours for TPN and 4.8 (SD, 4.9) hours for IV (Figure 1). In the past 6 months, 13.4% experienced ≥1 central line infection or sepsis, and 7.3% had thrombosis (Figure 2). Additionally, 41.8% had an emergency department visit, and 27.5% were hospitalized overnight (mean duration, 10.2 nights).
Discussion: Adults with SBS requiring PS report high rates of debilitating symptoms, serious complications, and intensive PS requirements. Despite current therapies, many experienced fatigue, abdominal pain, malabsorption, and dehydration. Anxiety, depression, and serious PS-related complications, such as sepsis and thrombosis, also were common. These findings highlight the urgent need for more effective interventions to reduce physical and psychological burden for the SBS population.
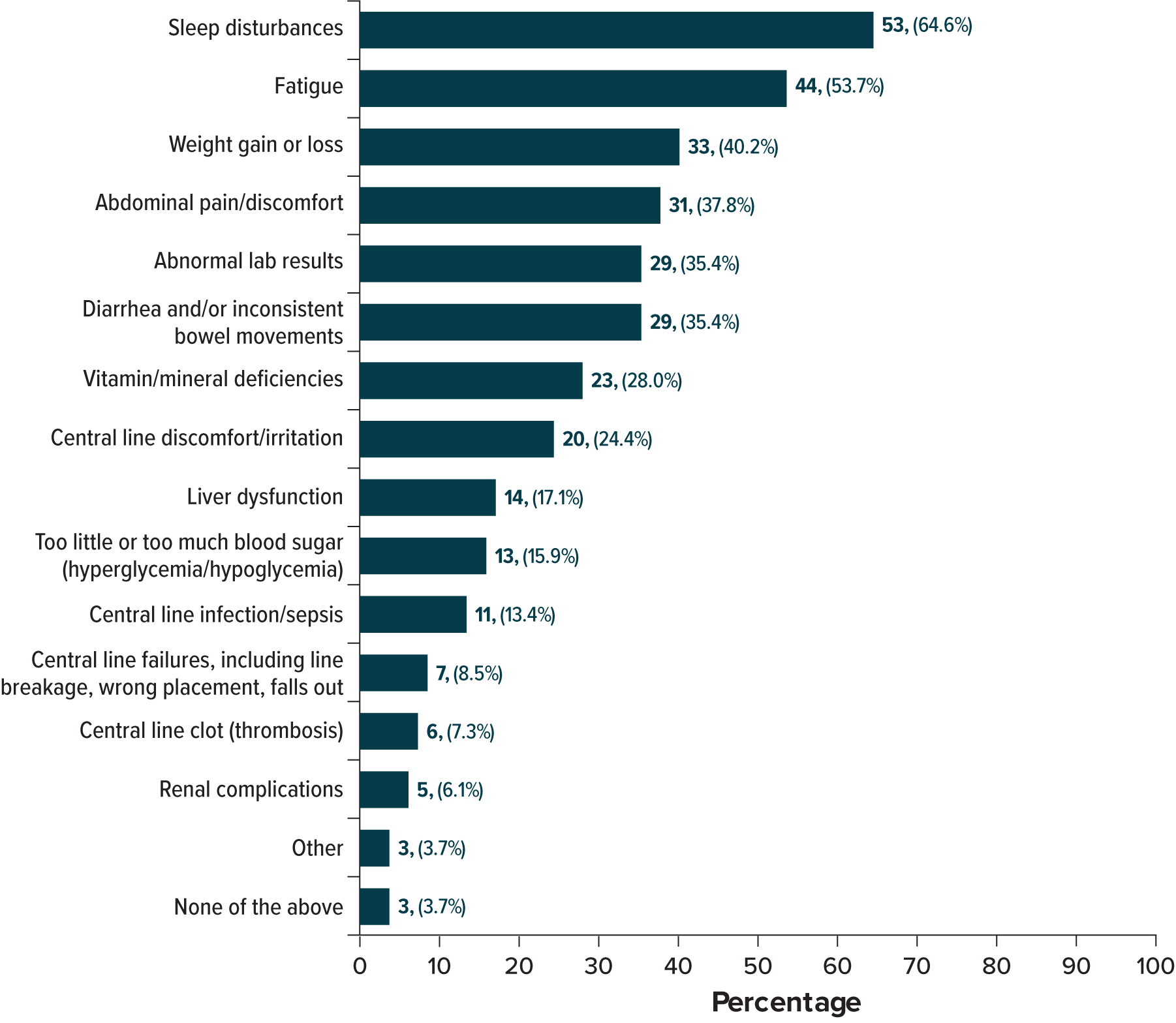
Figure: Figure 1. Use of TPN and IV Hydration
IV, intravenous; TPN, total parenteral nutrition
Disclosures:
Marion Winkler: American Regent – Consultant. Ironwood Pharmaceutical – Consultant. Takeda Pharmaceutical – Consultant.
Jenny Harrison indicated no relevant financial relationships.
Roopa Vemulapalli indicated no relevant financial relationships.
Vanessa Kumpf: American Regent – Consultant. Baxter Healthcare – Consultant. Fresenius Kabi – Consultant. VectivBio (now Ironwood) – Consultant.
Gail Mitchell: ironwood pharmaceuticals – Consultant.
Daniel Wolin indicated no relevant financial relationships.
Laurie Zografos indicated no relevant financial relationships.
Mindy Yang: Ironwood Pharmaceuticals – Consultant.
Laurin Jackson indicated no relevant financial relationships.
Jinyi Wang indicated no relevant financial relationships.
Jeff Henderson: Ironwood Pharmaceuticals – Employee.
Mena Boules: Ironwood Pharmaceuticals, Inc (Previously VectivBio) – Employee, Stock-publicly held company(excluding mutual/index funds).
Syed-Mohammed Jafri: Abbvie – Speakers Bureau. Gilead – Speakers Bureau. Intercept – Speakers Bureau. Ironwood – Speakers Bureau. Takeda – Speakers Bureau.
Marion F.. Winkler, PhD, RD1, Jenny E.. Harrison, MS2, Roopa Vemulapalli, MD3, Vanessa J.. Kumpf, PharmD4, Gail Mitchell, MBA5, Daniel Wolin, BS6, Laurie Zografos, BS7, Mindy Yang, PharmD8, Laurin Jackson, MA7, Jinyi Wang, MS9, Jeff Henderson, BA10, Mena Boules, MD11, Syed-Mohammed Jafri, MD12. P4053 - Clinical and Psychological Burden Among Patients with Short Bowel Syndrome Requiring Parenteral Support: Findings From a Multinational Survey, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Rhode Island Hospital, Providence, RI; 2Girls With Guts, Turners Falls, MA; 3University of Texas Southwestern Medical Center, Dallas, TX; 4Vanderbilt University Medical Center, Nashville, TN; 5Ironwood Pharmaceuticals, Walchwil, Zug, Switzerland; 6RTI-Health Solutions, Marshall, MI; 7RTI Health Solutions, Research Triangle Park, NC; 8Ironwood Pharmaceuticals, Mount Pocono, PA; 9RTI-HS, Research Triangle Park, NC; 10Ironwood Pharmaceuticals, Jupiter, FL; 11Ironwood Pharmaceuticals, Inc., Boston, MA; 12Henry Ford Health, Detroit, MI
Introduction: Short bowel syndrome (SBS) is a rare, complex condition characterized by reduced intestinal absorptive capacity. Many patients require long-term parenteral support (PS), including total parenteral nutrition (TPN) and/or intravenous (IV) hydration, to maintain hydration and nutrition. Our study evaluated the clinical and psychological burden associated with SBS and its current management approaches.
Methods: In the context of a noninterventional, cross-sectional, online survey among patients with SBS in the United States (US) and Europe, adults (≥18 years) with SBS dependent on PS provided information on their comorbidities, symptoms or complications of SBS, healthcare resource utilization, and PS use and associated symptoms.
Results: Among 91 patients (50 from the US; 41 from Europe), 69.1% were White, 58.2% were female, and the mean age at SBS diagnosis was 43.5 years. The majority (79.3%-80.6%) reported using TPN and/or IV hydration for over 1 year. Over half (52.7%) reported a stoma/ostomy bag, and 27.8% had a feeding tube. Common comorbidities included Crohn’s disease (25.3%), fistula (13.2%), and ulcerative colitis (11.0%). In the past 6 months, patients frequently reported experiencing fatigue (73.6%), abdominal pain/discomfort (60.4%), anxiety/depression (59.3%), malabsorption/weight loss (52.7%), and dehydration/electrolyte imbalances (51.6%).
All patients were PS dependent: 26.4% on TPN only, 9.9% on IV hydration only, and 63.7% on both; 24.2% reported current glucagon-like peptide-2 analog use. PS was infused frequently: 72.0% and 52.2% received TPN or IV hydration ≥5 days per week, respectively. Daily treatment duration averaged 10.1 (standard deviation [SD], 4.2) hours for TPN and 4.8 (SD, 4.9) hours for IV (Figure 1). In the past 6 months, 13.4% experienced ≥1 central line infection or sepsis, and 7.3% had thrombosis (Figure 2). Additionally, 41.8% had an emergency department visit, and 27.5% were hospitalized overnight (mean duration, 10.2 nights).
Discussion: Adults with SBS requiring PS report high rates of debilitating symptoms, serious complications, and intensive PS requirements. Despite current therapies, many experienced fatigue, abdominal pain, malabsorption, and dehydration. Anxiety, depression, and serious PS-related complications, such as sepsis and thrombosis, also were common. These findings highlight the urgent need for more effective interventions to reduce physical and psychological burden for the SBS population.

Figure: Figure 1. Use of TPN and IV Hydration
IV, intravenous; TPN, total parenteral nutrition

Disclosures:
Marion Winkler: American Regent – Consultant. Ironwood Pharmaceutical – Consultant. Takeda Pharmaceutical – Consultant.
Jenny Harrison indicated no relevant financial relationships.
Roopa Vemulapalli indicated no relevant financial relationships.
Vanessa Kumpf: American Regent – Consultant. Baxter Healthcare – Consultant. Fresenius Kabi – Consultant. VectivBio (now Ironwood) – Consultant.
Gail Mitchell: ironwood pharmaceuticals – Consultant.
Daniel Wolin indicated no relevant financial relationships.
Laurie Zografos indicated no relevant financial relationships.
Mindy Yang: Ironwood Pharmaceuticals – Consultant.
Laurin Jackson indicated no relevant financial relationships.
Jinyi Wang indicated no relevant financial relationships.
Jeff Henderson: Ironwood Pharmaceuticals – Employee.
Mena Boules: Ironwood Pharmaceuticals, Inc (Previously VectivBio) – Employee, Stock-publicly held company(excluding mutual/index funds).
Syed-Mohammed Jafri: Abbvie – Speakers Bureau. Gilead – Speakers Bureau. Intercept – Speakers Bureau. Ironwood – Speakers Bureau. Takeda – Speakers Bureau.
Marion F.. Winkler, PhD, RD1, Jenny E.. Harrison, MS2, Roopa Vemulapalli, MD3, Vanessa J.. Kumpf, PharmD4, Gail Mitchell, MBA5, Daniel Wolin, BS6, Laurie Zografos, BS7, Mindy Yang, PharmD8, Laurin Jackson, MA7, Jinyi Wang, MS9, Jeff Henderson, BA10, Mena Boules, MD11, Syed-Mohammed Jafri, MD12. P4053 - Clinical and Psychological Burden Among Patients with Short Bowel Syndrome Requiring Parenteral Support: Findings From a Multinational Survey, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
