Monday Poster Session
Category: Small Intestine
P4045 - Comparative Risk of Small Intestinal Bacterial Overgrowth (SIBO) Among Patients Using GLP-1 Receptor Agonists: A Real-World Analysis Using TriNetX
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Lina AlQirem, MD
University of Arkansas for Medical Sciences
Little Rock, AR
Presenting Author(s)
Lina AlQirem, MD1, Suria Devarapalli, DO1, Adalberto Guzman, MD2, Murad Qirem, MD3, Meer A. Ali, MD1
1University of Arkansas for Medical Sciences, Little Rock, AR; 2University of Arkansas, Little Rock, AR; 3New York Medical College - Saint Michael's Medical Center, West Orange, NJ
Introduction: Glucagon-like peptide-1 (GLP-1) is a gut-derived incretin hormone, offering benefits such as weight loss and improved glycemic control. These agents slow gastric emptying and intestinal transit, which theoretically could predispose to small intestinal bacterial overgrowth (SIBO). However, there is no defining link to establish GLP-1 receptor agonist agents as a direct cause of SIBO. Our study aims to explore the relationship between GLP-1 RA and the development of SIBO.
Methods: A retrospective cohort study was conducted using the TriNetX Research Network, a federated database comprising de-identified electronic health records from multiple healthcare organizations. Adult patients prescribed a GLP-1 RA (albiglutide, dulaglutide, exenatide, liraglutide, lixisenatide, semaglutide, or tirzepatide) between January 2010 and May 2025 were included. Patients with prior diagnoses or procedures known to affect gastrointestinal motility (e.g. gastroparesis, bowel resections), and conditions that could interfere with breath testing accuracy (e.g., lactose intolerance, fructose intolerance) were excluded. The primary outcome was a new diagnosis of SIBO following GLP-1 RA exposure. Incidence rates were calculated for each agent individually.
Results: A total of 2,305,630 patients were analyzed. The incidence of SIBO varied across the different GLP-1 receptor agonist agents. Albiglutide demonstrated the highest risk (0.156%), while tirzepatide showed the lowest (0.022%). Intermediate risks were observed for exenatide (0.048%), dulaglutide (0.047%), liraglutide (0.055%), and lixisenatide (0.073%). Semaglutide, despite the largest cohort (n=992,660), had a relatively low risk (0.039%). Kaplan-Meier analysis confirmed temporal consistency (p< 0.05 for all agents).
Discussion: The overall risk of developing SIBO among GLP-1 receptor agonist users was low but variable across agents. This variability in SIBO risk among GLP-1 agonists, reflects potential differences in pharmacokinetics (e.g., albiglutide's weekly administration vs. exenatide's shorter half-life). The elevated risk with albiglutide and lixisenatide may relate to prolonged gastric emptying effects, while trizepatide's GIP/GLP-1 dual agonism might offer protective mechanisms. Further studies are warranted to better characterize this potential adverse effect and guide agent selection in patients at risk for SIBO.
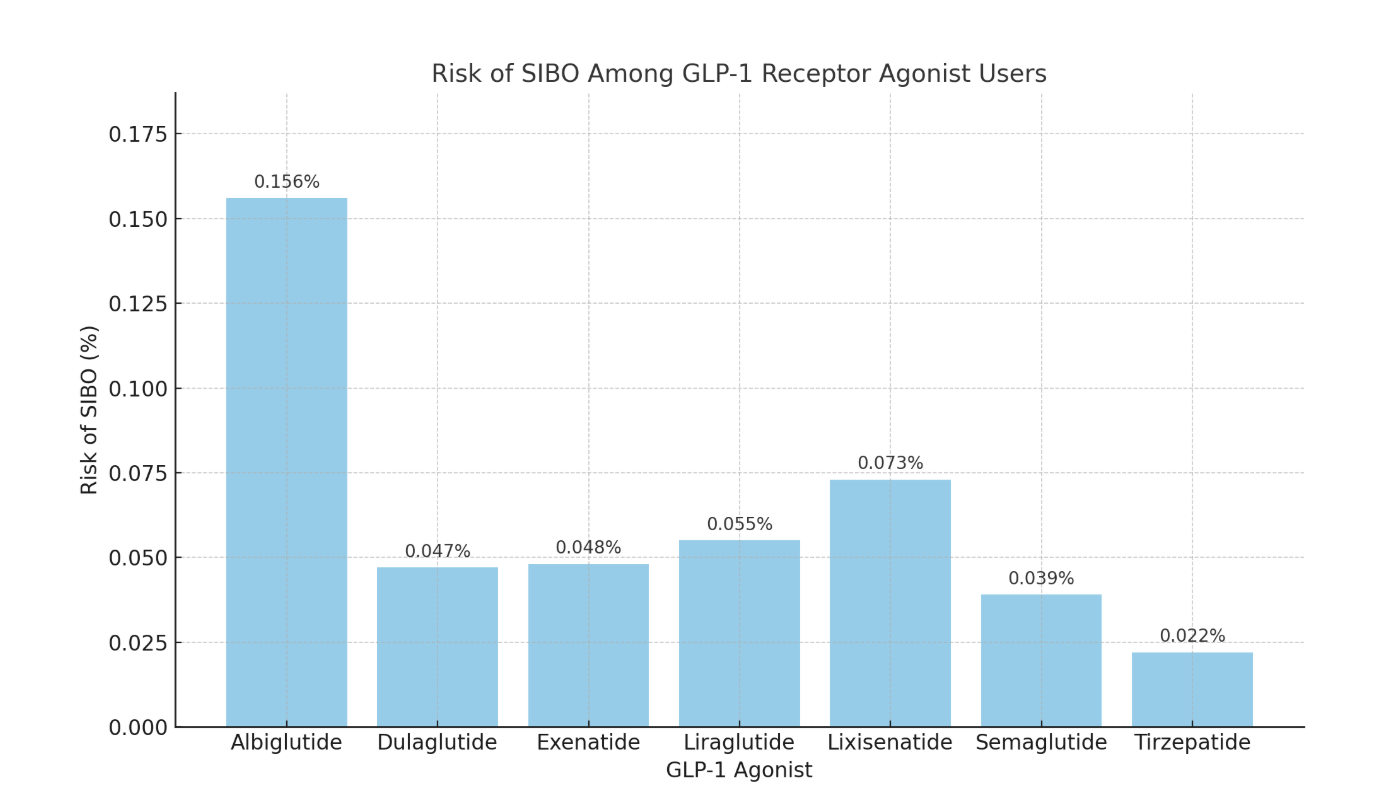
Figure: Graph showing the risk of SIBO with different GLP-RA agents
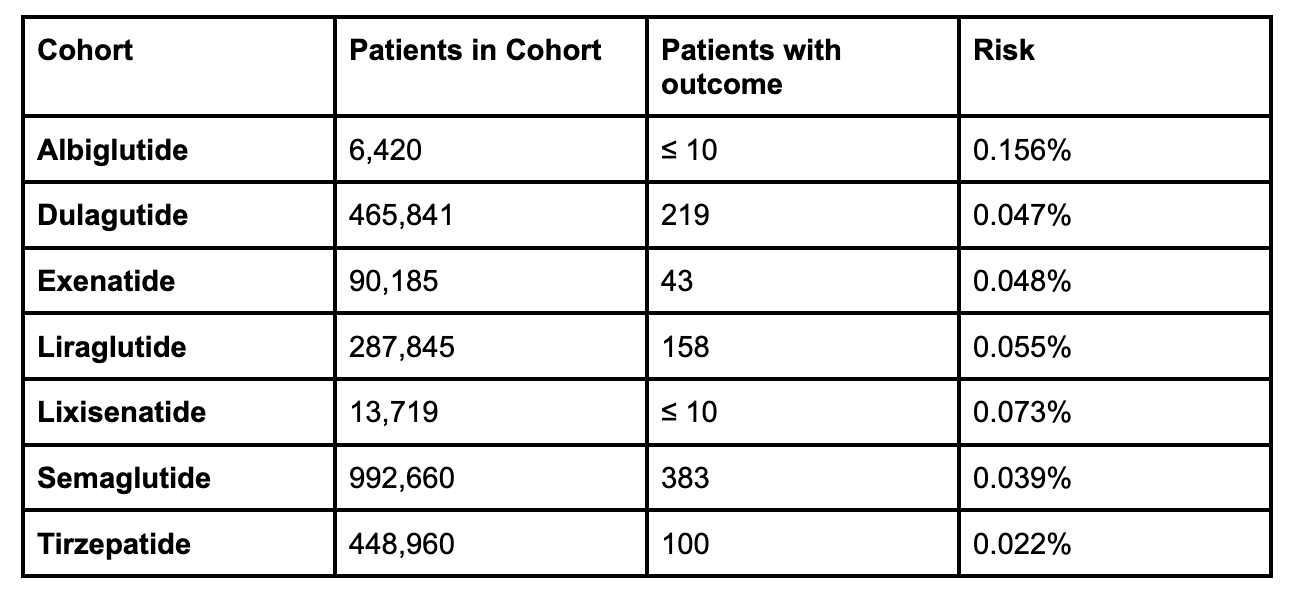
Figure: Table showing the risk of SIBO with different GLP-1 RA agents
Disclosures:
Lina AlQirem indicated no relevant financial relationships.
Suria Devarapalli indicated no relevant financial relationships.
Adalberto Guzman indicated no relevant financial relationships.
Murad Qirem indicated no relevant financial relationships.
Meer Ali indicated no relevant financial relationships.
Lina AlQirem, MD1, Suria Devarapalli, DO1, Adalberto Guzman, MD2, Murad Qirem, MD3, Meer A. Ali, MD1. P4045 - Comparative Risk of Small Intestinal Bacterial Overgrowth (SIBO) Among Patients Using GLP-1 Receptor Agonists: A Real-World Analysis Using TriNetX, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Arkansas for Medical Sciences, Little Rock, AR; 2University of Arkansas, Little Rock, AR; 3New York Medical College - Saint Michael's Medical Center, West Orange, NJ
Introduction: Glucagon-like peptide-1 (GLP-1) is a gut-derived incretin hormone, offering benefits such as weight loss and improved glycemic control. These agents slow gastric emptying and intestinal transit, which theoretically could predispose to small intestinal bacterial overgrowth (SIBO). However, there is no defining link to establish GLP-1 receptor agonist agents as a direct cause of SIBO. Our study aims to explore the relationship between GLP-1 RA and the development of SIBO.
Methods: A retrospective cohort study was conducted using the TriNetX Research Network, a federated database comprising de-identified electronic health records from multiple healthcare organizations. Adult patients prescribed a GLP-1 RA (albiglutide, dulaglutide, exenatide, liraglutide, lixisenatide, semaglutide, or tirzepatide) between January 2010 and May 2025 were included. Patients with prior diagnoses or procedures known to affect gastrointestinal motility (e.g. gastroparesis, bowel resections), and conditions that could interfere with breath testing accuracy (e.g., lactose intolerance, fructose intolerance) were excluded. The primary outcome was a new diagnosis of SIBO following GLP-1 RA exposure. Incidence rates were calculated for each agent individually.
Results: A total of 2,305,630 patients were analyzed. The incidence of SIBO varied across the different GLP-1 receptor agonist agents. Albiglutide demonstrated the highest risk (0.156%), while tirzepatide showed the lowest (0.022%). Intermediate risks were observed for exenatide (0.048%), dulaglutide (0.047%), liraglutide (0.055%), and lixisenatide (0.073%). Semaglutide, despite the largest cohort (n=992,660), had a relatively low risk (0.039%). Kaplan-Meier analysis confirmed temporal consistency (p< 0.05 for all agents).
Discussion: The overall risk of developing SIBO among GLP-1 receptor agonist users was low but variable across agents. This variability in SIBO risk among GLP-1 agonists, reflects potential differences in pharmacokinetics (e.g., albiglutide's weekly administration vs. exenatide's shorter half-life). The elevated risk with albiglutide and lixisenatide may relate to prolonged gastric emptying effects, while trizepatide's GIP/GLP-1 dual agonism might offer protective mechanisms. Further studies are warranted to better characterize this potential adverse effect and guide agent selection in patients at risk for SIBO.

Figure: Graph showing the risk of SIBO with different GLP-RA agents

Figure: Table showing the risk of SIBO with different GLP-1 RA agents
Disclosures:
Lina AlQirem indicated no relevant financial relationships.
Suria Devarapalli indicated no relevant financial relationships.
Adalberto Guzman indicated no relevant financial relationships.
Murad Qirem indicated no relevant financial relationships.
Meer Ali indicated no relevant financial relationships.
Lina AlQirem, MD1, Suria Devarapalli, DO1, Adalberto Guzman, MD2, Murad Qirem, MD3, Meer A. Ali, MD1. P4045 - Comparative Risk of Small Intestinal Bacterial Overgrowth (SIBO) Among Patients Using GLP-1 Receptor Agonists: A Real-World Analysis Using TriNetX, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
