Monday Poster Session
Category: Liver
P3920 - Not All That’s Herbal Is Healthy: A Case of Drug-Induced Liver Injury With Autoimmune Features from Turkesterone
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Arvin N. Tan, MD
University of Hawaii, John A. Burns School of Medicine, Department of Medicine
Honolulu, HI
Presenting Author(s)
Arvin Jeremy N. Tan, MD1, Slaton Case, MD1, Akiko Tokunaga, MD2, Linda Wong, MD3, Tomoki Sempokuya, MD1
1University of Hawaii, John A. Burns School of Medicine, Department of Medicine, Honolulu, HI; 2University of Hawaii, John A. Burns School of Medicine, Department of Pathology, Honolulu, HI; 3University of Hawaii, John A. Burns School of Medicine, Department of Surgery, Honolulu, HI
Introduction: Drug-induced liver injury (DILI) is a leading cause of acute liver injury in the United States, with an annual incidence of 15 to 20 per 100,000. Serologic and histologic features of autoimmune hepatitis (AIH) may present with DILI and cause diagnostic uncertainty. Here, we report a case of a patient with Turkesterone-related DILI with autoimmune features.
Case Description/
Methods: A 39-year-old male with a history of subfulminant liver failure post liver transplantation in 2011 presented to the emergency department with a three-week history of generalized weakness, poor appetite, nausea, and dark urine. Laboratory testing revealed elevated liver enzymes compared to 10 months prior, which were within normal limits (Figure 1). Liver ultrasound showed patent portal and hepatic veins, and a computed tomography (CT) scan of the abdomen and pelvis revealed no pathology. Tests for viral hepatitis, cytomegalovirus (CMV), and antinuclear antibodies (ANA) were negative. Serology revealed elevated IgG levels at 1800 mg/dL, and positive anti-smooth muscle antibodies (ASMA) at 38 U. His liver-kidney microsomal antibodies (LKM) were negative. He later revealed that he has been taking a supplement of Turkesterone for the past two months. Liver biopsy demonstrated lymphoplasmacytic portal inflammation with interface activity consistent with autoimmune hepatitis (AIH) or DILI (Image 1A-1B). The patient was treated with 500 mg of methylprednisolone, with rapid improvement of his symptoms. He was discharged on a prednisone taper with recovery of his liver enzymes at two-month follow-up.
Discussion: Turkesterone is part of the ecdysteroid family, a group of plant hormones studied for their anabolic effects. Data on its safety is limited. Our patient was taking approximately 160 mg of turkesterone, far exceeding the doses investigated in available studies. The temporal relationship of the patient’s supplement intake, onset of liver injury, histologic findings on biopsy, and subsequent improvement with glucocorticoid therapy strongly suggests turkesterone-related DILI with autoimmune features. While drugs like nitrofurantoin, minocycline, hydralazine, and methyldopa are known to cause DILI with autoimmune features, cases implicating herbal supplements are rare. This case highlights the importance of a thorough review of the use of herbal and dietary supplements with patients.
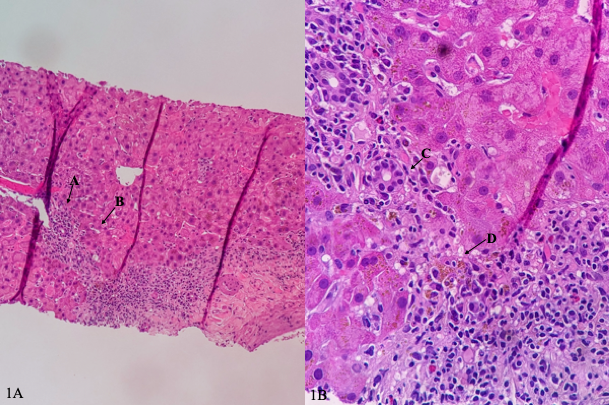
Figure: Figure 1. Liver enzymes, tacrolimus levels, coagulation profile, and platelet count from initial presentation to follow-up.
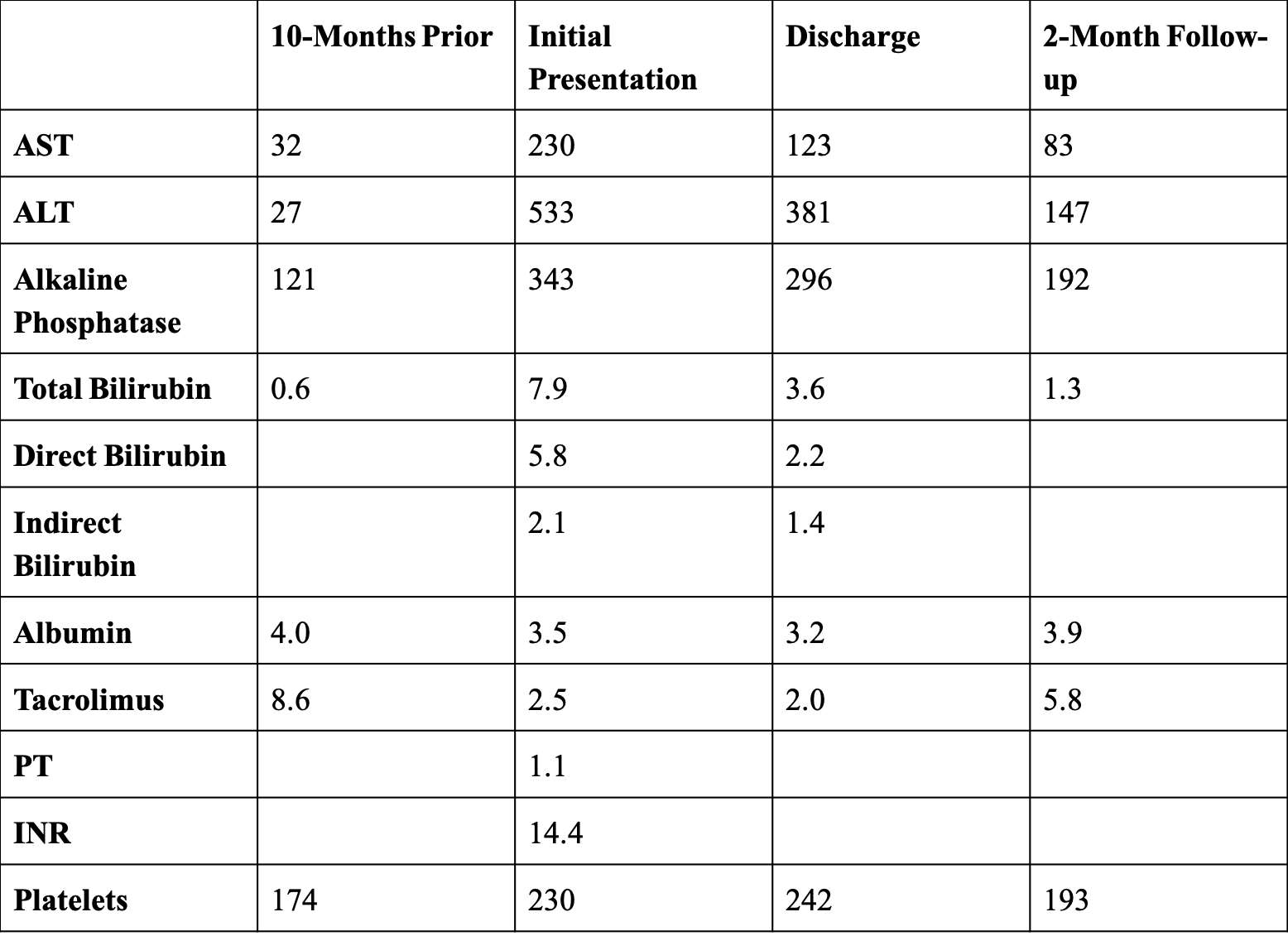
Figure: Image 1A-1B: Photomicrograph of liver biopsy specimen demonstrating lymphoplasmacytic portal inflammation (arrow A), interface activity or inflammation extending beyond the portal tract and crossing the limiting plate (arrow B), ductulitis (arrow C), and cholestasis (arrow D).
Disclosures:
Arvin Jeremy Tan indicated no relevant financial relationships.
Slaton Case indicated no relevant financial relationships.
Akiko Tokunaga indicated no relevant financial relationships.
Linda Wong: Astra Zeneca – Speakers Bureau.
Tomoki Sempokuya indicated no relevant financial relationships.
Arvin Jeremy N. Tan, MD1, Slaton Case, MD1, Akiko Tokunaga, MD2, Linda Wong, MD3, Tomoki Sempokuya, MD1. P3920 - Not All That’s Herbal Is Healthy: A Case of Drug-Induced Liver Injury With Autoimmune Features from Turkesterone, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Hawaii, John A. Burns School of Medicine, Department of Medicine, Honolulu, HI; 2University of Hawaii, John A. Burns School of Medicine, Department of Pathology, Honolulu, HI; 3University of Hawaii, John A. Burns School of Medicine, Department of Surgery, Honolulu, HI
Introduction: Drug-induced liver injury (DILI) is a leading cause of acute liver injury in the United States, with an annual incidence of 15 to 20 per 100,000. Serologic and histologic features of autoimmune hepatitis (AIH) may present with DILI and cause diagnostic uncertainty. Here, we report a case of a patient with Turkesterone-related DILI with autoimmune features.
Case Description/
Methods: A 39-year-old male with a history of subfulminant liver failure post liver transplantation in 2011 presented to the emergency department with a three-week history of generalized weakness, poor appetite, nausea, and dark urine. Laboratory testing revealed elevated liver enzymes compared to 10 months prior, which were within normal limits (Figure 1). Liver ultrasound showed patent portal and hepatic veins, and a computed tomography (CT) scan of the abdomen and pelvis revealed no pathology. Tests for viral hepatitis, cytomegalovirus (CMV), and antinuclear antibodies (ANA) were negative. Serology revealed elevated IgG levels at 1800 mg/dL, and positive anti-smooth muscle antibodies (ASMA) at 38 U. His liver-kidney microsomal antibodies (LKM) were negative. He later revealed that he has been taking a supplement of Turkesterone for the past two months. Liver biopsy demonstrated lymphoplasmacytic portal inflammation with interface activity consistent with autoimmune hepatitis (AIH) or DILI (Image 1A-1B). The patient was treated with 500 mg of methylprednisolone, with rapid improvement of his symptoms. He was discharged on a prednisone taper with recovery of his liver enzymes at two-month follow-up.
Discussion: Turkesterone is part of the ecdysteroid family, a group of plant hormones studied for their anabolic effects. Data on its safety is limited. Our patient was taking approximately 160 mg of turkesterone, far exceeding the doses investigated in available studies. The temporal relationship of the patient’s supplement intake, onset of liver injury, histologic findings on biopsy, and subsequent improvement with glucocorticoid therapy strongly suggests turkesterone-related DILI with autoimmune features. While drugs like nitrofurantoin, minocycline, hydralazine, and methyldopa are known to cause DILI with autoimmune features, cases implicating herbal supplements are rare. This case highlights the importance of a thorough review of the use of herbal and dietary supplements with patients.

Figure: Figure 1. Liver enzymes, tacrolimus levels, coagulation profile, and platelet count from initial presentation to follow-up.

Figure: Image 1A-1B: Photomicrograph of liver biopsy specimen demonstrating lymphoplasmacytic portal inflammation (arrow A), interface activity or inflammation extending beyond the portal tract and crossing the limiting plate (arrow B), ductulitis (arrow C), and cholestasis (arrow D).
Disclosures:
Arvin Jeremy Tan indicated no relevant financial relationships.
Slaton Case indicated no relevant financial relationships.
Akiko Tokunaga indicated no relevant financial relationships.
Linda Wong: Astra Zeneca – Speakers Bureau.
Tomoki Sempokuya indicated no relevant financial relationships.
Arvin Jeremy N. Tan, MD1, Slaton Case, MD1, Akiko Tokunaga, MD2, Linda Wong, MD3, Tomoki Sempokuya, MD1. P3920 - Not All That’s Herbal Is Healthy: A Case of Drug-Induced Liver Injury With Autoimmune Features from Turkesterone, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
