Monday Poster Session
Category: Liver
P3915 - Déjà Vu! A Case of Recurrent MASH 60 Days After Liver Transplantation
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Tanner Thornsberry, MD
University of Cincinnati Medical Center
Cincinnati, OH
Presenting Author(s)
Award: ACG Presidential Poster Award
Tanner Thornsberry, MD1, Jacob Circillo, MD2, Divya Sharma, MD1, Alana Hofmann, MD1, Ralph C. Quillin III, MD1, Christine Haugen, MD, PhD1, Adam Myer, MD2, Amoah Yeboah-Korang, MD3
1University of Cincinnati Medical Center, Cincinnati, OH; 2University of Cincinnati College of Medicine, Cincinnati, OH; 3University of Cincinnati, Cincinnati, OH
Introduction: Metabolic dysfunction-associated steatotic liver disease (MASLD) is the leading indication for liver transplantation (LT) among women and those older than 65 years. Recurrent MASLD after LT was about 5-fold higher than developing de novo disease. Although incidence of MASLD recurrence increases with time after LT with < 20% rate of progression to MASH, early (< 12 months) MASH recurrence is rare, but associated with higher incidence of advance fibrosis (~ 40%), negatively impacting graft and patient survival. We present a rare case of MASH recurrence within 10 weeks after LT.
Case Description/
Methods: A forty-year-old woman with obesity s/p gastric sleeve, sleep apnea, kidney transplant complicated by tacrolimus-induced microangiopathy, and cirrhosis secondary to MASH who had LT two months prior presented for follow up. Initial biopsy of allograft showed 5% macrosteatosis and no fibrosis. Current immunosuppression: mycophenolate, prednisone and belatacept. Labs showed AST 73 and ALT 99 compared to 34 and 48 respectively two weeks prior. Liver ultrasound demonstrated echogenicity of the transplanted liver suggestive of steatosis. Subsequent liver biopsy showed moderate acute cellular rejection (ACR), and severe microvesicular steaosis (68%) without significant fibrosis (F0). Pulse dose steroids per protocol were started for ACR; however, her liver tests increased to peak AST 180 and ALT 272. Repeat liver biopsy 6 days later demonstrated severe steatosis ( >95%), with ballooning hepatocytes consistent with active steatohepatitis, no evidence of ACR, and patchy pericellular fibrosis (F1). With confirmed MASH recurrence, she was started on a GLP-1 agonist, fish oil, and vitamin E. Liver tests significantly improved to AST and ALT in the 60s over the next 60 days.
Discussion: This case highlights how recurrent MASLD can rapidly progress to MASH in susceptible LT recipients. Multiple factors such as genetics, diet, weight gain, high doses of steroids and other immunosuppression in the immediate post-LT period likely contributed to the early recurrence of MASLD and progression to MASH. Risk stratification tools to identify those at risk for early MASLD recurrence and progression post-LT as well as interventions to aggressively manage modifiable risk factors will likely improve graft and patient survival. Although there is no FDA approved treatment of MASH recurrence post-LT, this case shows good response to treatment with combination of GLP-1 agonists, an antioxidant, and omega-3 supplement.
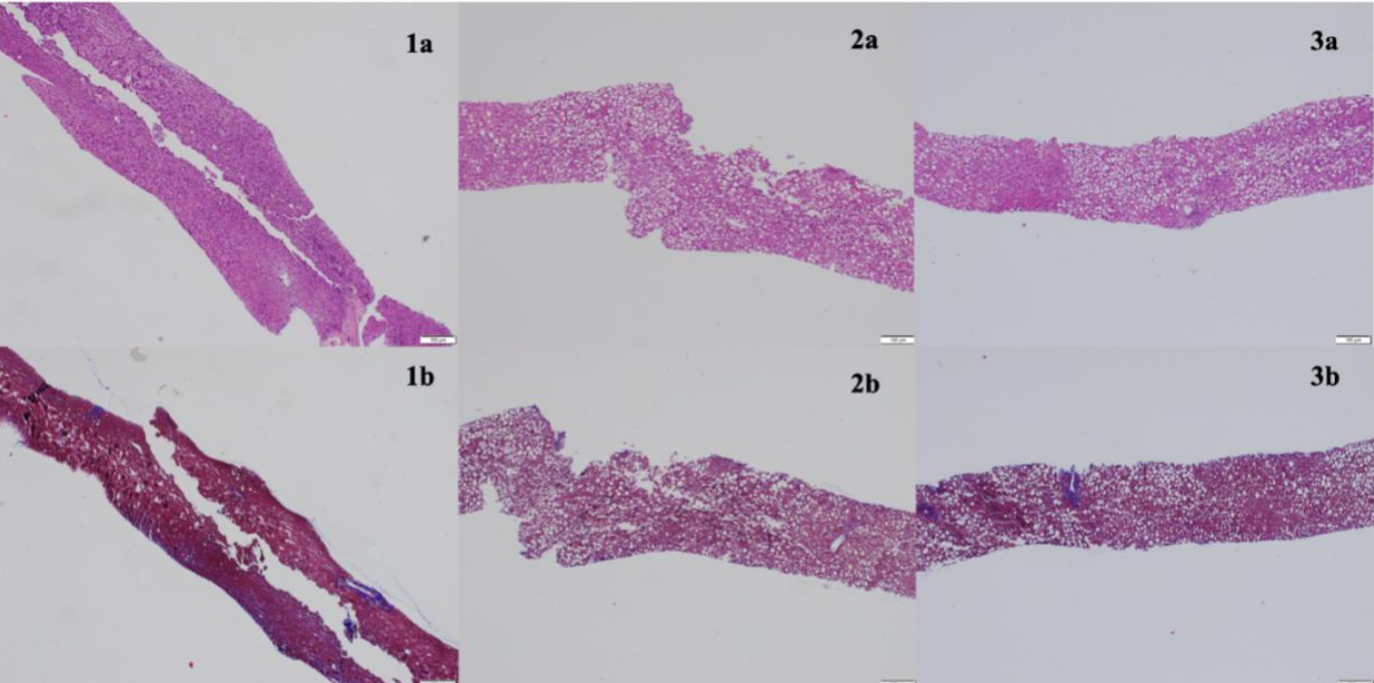
Figure: Figure 1: Initial biopsy at time of transplant (1a) with no steatosis present and associated trichrome stain (1b). First biopsy upon initial presentation (2a) with associated trichrome stain (2b) demonstrating >70% steatosis. Follow up biopsy demonstrating >95% steatosis with steatohepatitis (3a) and associated trichrome stain (3b).
Disclosures:
Tanner Thornsberry indicated no relevant financial relationships.
Jacob Circillo indicated no relevant financial relationships.
Divya Sharma indicated no relevant financial relationships.
Alana Hofmann indicated no relevant financial relationships.
Ralph Quillin III indicated no relevant financial relationships.
Christine Haugen indicated no relevant financial relationships.
Adam Myer indicated no relevant financial relationships.
Amoah Yeboah-Korang indicated no relevant financial relationships.
Tanner Thornsberry, MD1, Jacob Circillo, MD2, Divya Sharma, MD1, Alana Hofmann, MD1, Ralph C. Quillin III, MD1, Christine Haugen, MD, PhD1, Adam Myer, MD2, Amoah Yeboah-Korang, MD3. P3915 - Déjà Vu! A Case of Recurrent MASH 60 Days After Liver Transplantation, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Tanner Thornsberry, MD1, Jacob Circillo, MD2, Divya Sharma, MD1, Alana Hofmann, MD1, Ralph C. Quillin III, MD1, Christine Haugen, MD, PhD1, Adam Myer, MD2, Amoah Yeboah-Korang, MD3
1University of Cincinnati Medical Center, Cincinnati, OH; 2University of Cincinnati College of Medicine, Cincinnati, OH; 3University of Cincinnati, Cincinnati, OH
Introduction: Metabolic dysfunction-associated steatotic liver disease (MASLD) is the leading indication for liver transplantation (LT) among women and those older than 65 years. Recurrent MASLD after LT was about 5-fold higher than developing de novo disease. Although incidence of MASLD recurrence increases with time after LT with < 20% rate of progression to MASH, early (< 12 months) MASH recurrence is rare, but associated with higher incidence of advance fibrosis (~ 40%), negatively impacting graft and patient survival. We present a rare case of MASH recurrence within 10 weeks after LT.
Case Description/
Methods: A forty-year-old woman with obesity s/p gastric sleeve, sleep apnea, kidney transplant complicated by tacrolimus-induced microangiopathy, and cirrhosis secondary to MASH who had LT two months prior presented for follow up. Initial biopsy of allograft showed 5% macrosteatosis and no fibrosis. Current immunosuppression: mycophenolate, prednisone and belatacept. Labs showed AST 73 and ALT 99 compared to 34 and 48 respectively two weeks prior. Liver ultrasound demonstrated echogenicity of the transplanted liver suggestive of steatosis. Subsequent liver biopsy showed moderate acute cellular rejection (ACR), and severe microvesicular steaosis (68%) without significant fibrosis (F0). Pulse dose steroids per protocol were started for ACR; however, her liver tests increased to peak AST 180 and ALT 272. Repeat liver biopsy 6 days later demonstrated severe steatosis ( >95%), with ballooning hepatocytes consistent with active steatohepatitis, no evidence of ACR, and patchy pericellular fibrosis (F1). With confirmed MASH recurrence, she was started on a GLP-1 agonist, fish oil, and vitamin E. Liver tests significantly improved to AST and ALT in the 60s over the next 60 days.
Discussion: This case highlights how recurrent MASLD can rapidly progress to MASH in susceptible LT recipients. Multiple factors such as genetics, diet, weight gain, high doses of steroids and other immunosuppression in the immediate post-LT period likely contributed to the early recurrence of MASLD and progression to MASH. Risk stratification tools to identify those at risk for early MASLD recurrence and progression post-LT as well as interventions to aggressively manage modifiable risk factors will likely improve graft and patient survival. Although there is no FDA approved treatment of MASH recurrence post-LT, this case shows good response to treatment with combination of GLP-1 agonists, an antioxidant, and omega-3 supplement.

Figure: Figure 1: Initial biopsy at time of transplant (1a) with no steatosis present and associated trichrome stain (1b). First biopsy upon initial presentation (2a) with associated trichrome stain (2b) demonstrating >70% steatosis. Follow up biopsy demonstrating >95% steatosis with steatohepatitis (3a) and associated trichrome stain (3b).
Disclosures:
Tanner Thornsberry indicated no relevant financial relationships.
Jacob Circillo indicated no relevant financial relationships.
Divya Sharma indicated no relevant financial relationships.
Alana Hofmann indicated no relevant financial relationships.
Ralph Quillin III indicated no relevant financial relationships.
Christine Haugen indicated no relevant financial relationships.
Adam Myer indicated no relevant financial relationships.
Amoah Yeboah-Korang indicated no relevant financial relationships.
Tanner Thornsberry, MD1, Jacob Circillo, MD2, Divya Sharma, MD1, Alana Hofmann, MD1, Ralph C. Quillin III, MD1, Christine Haugen, MD, PhD1, Adam Myer, MD2, Amoah Yeboah-Korang, MD3. P3915 - Déjà Vu! A Case of Recurrent MASH 60 Days After Liver Transplantation, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

